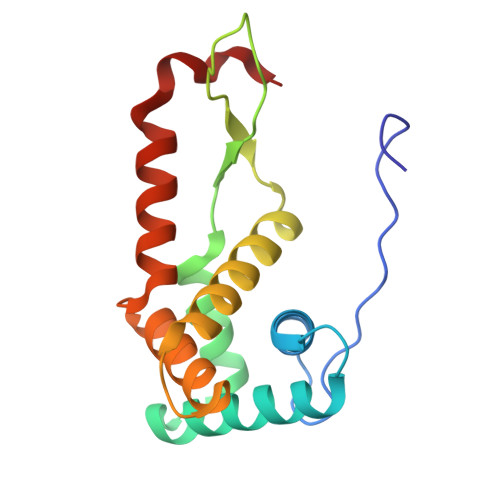
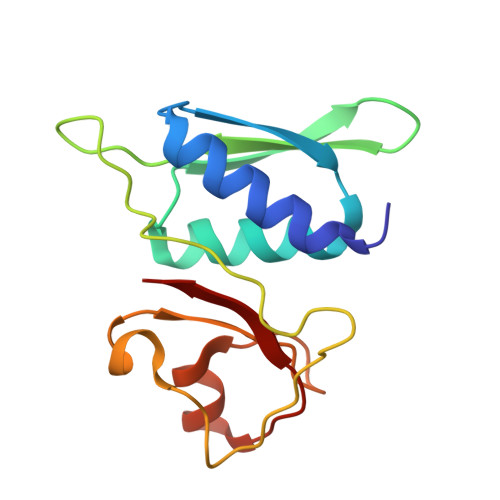
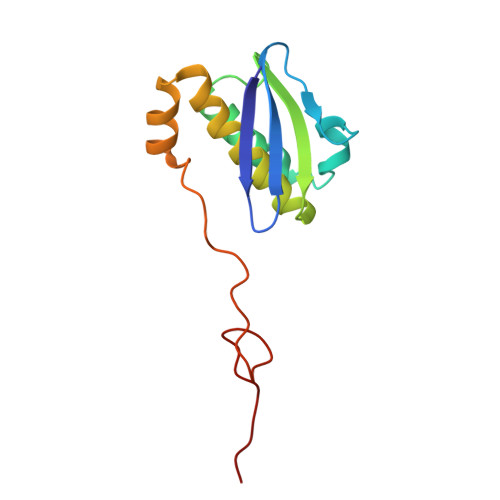
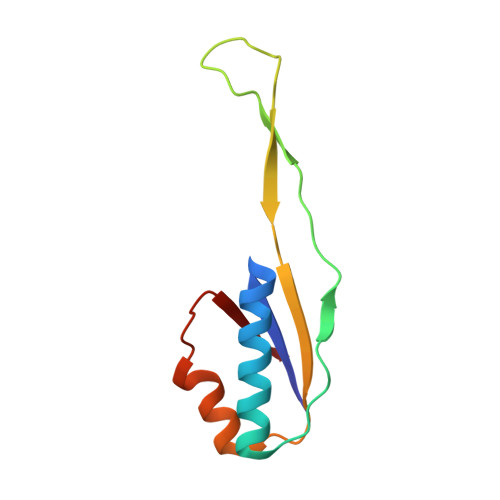
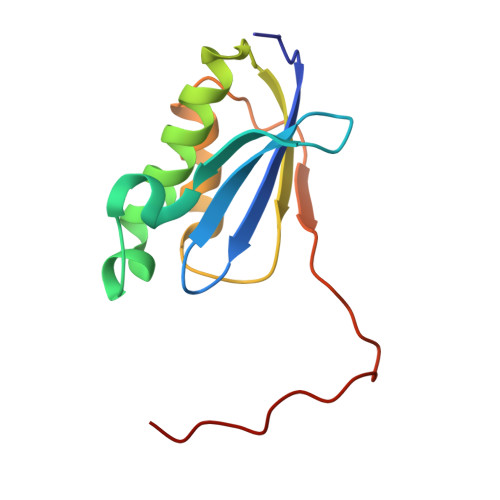
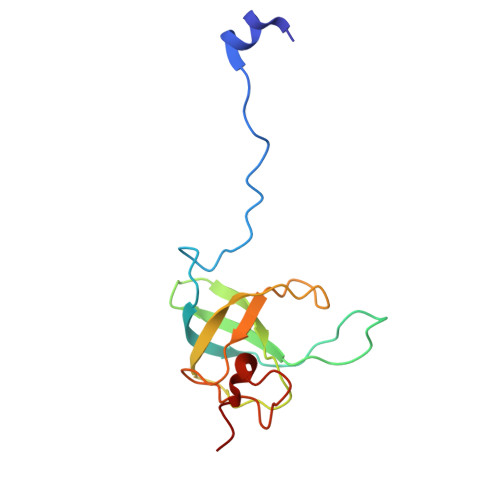
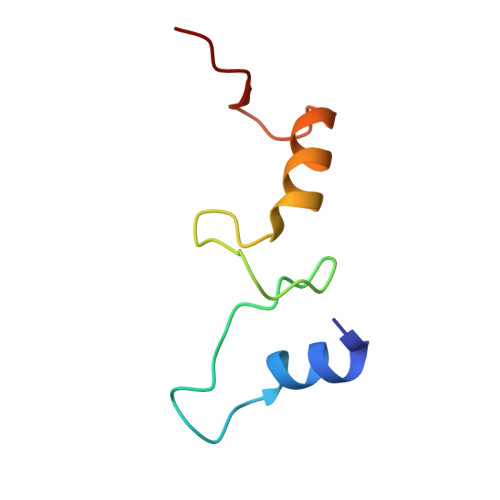
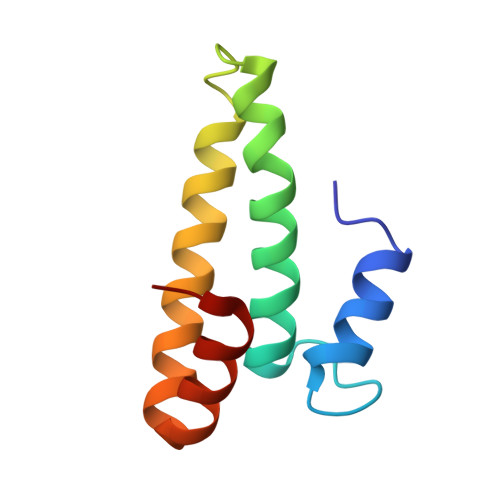
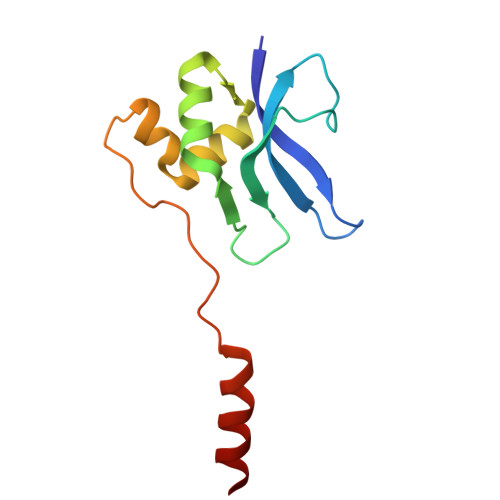
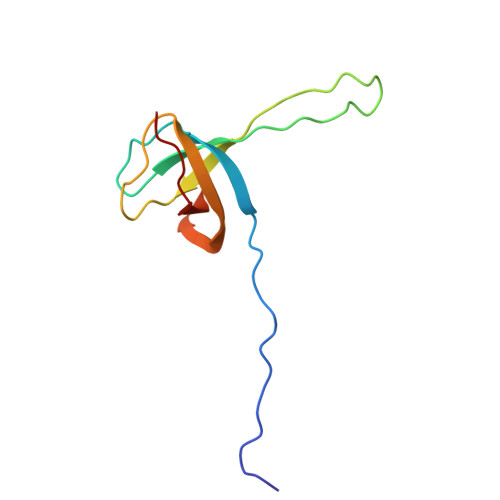
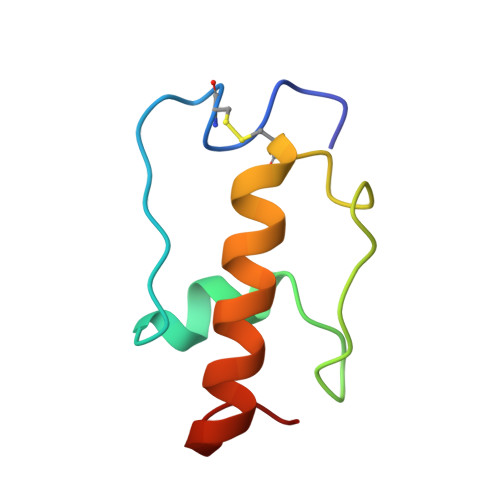
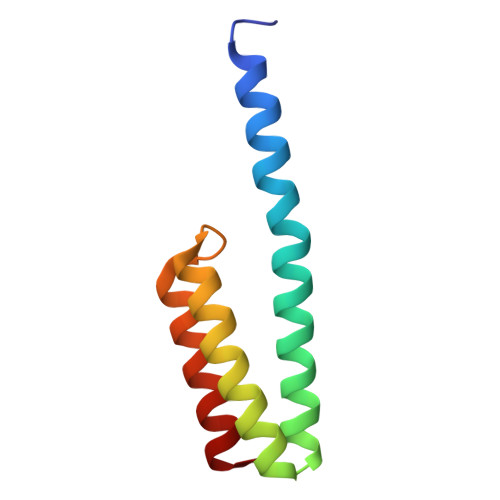
Mycobacterial Methionine Aminopeptidase Type 1c Moonlights as an Anti-association Factor on the 30S Ribosomal Subunit.
Banerjee, A., Srinivasan, K., Sengupta, J.(2025) J Mol Biology 437: 169230-169230
- PubMed: 40441414
- DOI: https://doi.org/10.1016/j.jmb.2025.169230
- Primary Citation of Related Structures:
8YP6 - PubMed Abstract:
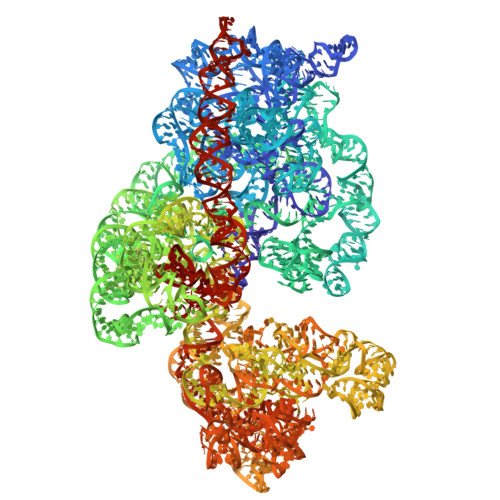
Methionine aminopeptidase (MetAP) is a vital metalloprotease that plays a crucial role in protein synthesis by binding to the 70S ribosome at the peptide exit tunnel and removing the N-terminal methionine from nascent polypeptide chains. In Escherichia coli, a single subclass of type 1 MetAP is present, whereas mycobacteria possess two subclasses, MetAP1a and MetAP1c. The key difference between these two is the presence of an additional 40 amino acid-long N-terminal extension in MetAP1c, which may contribute to distinct functional properties. In this study, we have uncovered a previously unrecognized "moonlighting" function of MetAP1c in mycobacteria. Interestingly, our results show that MetAP1c expression is specifically enhanced during the stationary phase of bacterial growth. Moreover, we identify a unique interaction between MetAP1c and the 30S ribosomal subunit, revealing its distinctive affinity for the small subunit. A 4.7 Å cryo-EM map of the Mycobacterium smegmatis MetAP1c-30S subunit complex demonstrates for the first time that MetAP1c binds at the inter-subunit face of the 30S subunit head region. The binding of MetAP1c induces conformational changes in the 30S subunit, impairing its ability to associate with the 50S subunit, thus imparting an anti-association property to MetAP1c. To further understand the role of the N-terminal extension, we constructed two mutant variants of MetAP1c, which confirmed its critical involvement in this moonlighting function. This anti-association activity of MetAP1c is likely one of the energy conservation mechanisms in mycobacteria where MetAP1c is involved in translation down regulation during stationary phase.
- Structural Biology and Bioinformatics Division, CSIR-Indian Institute of Chemical Biology, 4, Raja S.C. Mullick Road, Jadavpur, Kolkata 700032, India; Academy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, India.
Organizational Affiliation: