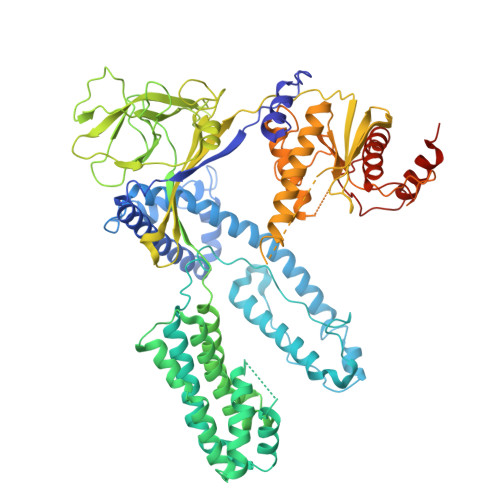
Cas12e orthologs evolve variable structural elements to facilitate dsDNA cleavage.
Li, D., Zhang, S., Lin, S., Xing, W., Yang, Y., Zhu, F., Su, D., Chen, C., Liu, J.G.(2024) Nat Commun 15: 10727-10727
- PubMed: 39737904
- DOI: https://doi.org/10.1038/s41467-024-54491-9
- Primary Citation of Related Structures:
8XYC, 8Y2I - PubMed Abstract:
Exceptionally diverse type V CRISPR-Cas systems provide numerous RNA-guided nucleases as powerful tools for DNA manipulation. Two known Cas12e nucleases, DpbCas12e and PlmCas12e, are both effective in genome editing. However, many differences exist in their in vitro dsDNA cleavage activities, reflecting the diversity in Cas12e's enzymatic properties. To comprehensively understand the Cas12e family, we identify and characterize six unreported Cas12e members that vary in their CRISPR-locus architectures, PAM preferences, and cleavage efficacies. Interestingly, among all variants, PlmCas12e exhibits the most robust trans-cleavage activity and the lowest salt sensitivity in cis-cleavage. Further structural comparisons reveal that the unique NTSB domain in PlmCas12e is beneficial to DNA unwinding at high salt concentrations, while some NTSB-lacking Cas12e proteins rely on positively charged loops for dsDNA unwinding. These findings demonstrate how divergent evolution of structural elements shapes the nuclease diversity within the Cas12e family, potentially contributing to their adaptations to varying environmental conditions.
- Beijing Frontier Research Center for Biological Structure, State Key Laboratory of Membrane Biology, School of Life Sciences, Tsinghua University, Beijing, 100084, China.
Organizational Affiliation: