Structural snapshots of human VMAT2 reveal insights into substrate recognition and proton coupling mechanism.
Wu, D., Yu, Z., Chen, Q., Zhao, J., Huang, B., Wang, Y., Su, J., Li, N., Jiang, D., Zhao, Y.(2024) Cell Res 34: 586-589
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Synaptic vesicular amine transporter,Synaptic vesicular amine transporter,transporterA | 573 | Homo sapiens | Mutation(s): 0 Gene Names: SLC18A2, SVMT, VMAT2 Membrane Entity: Yes | 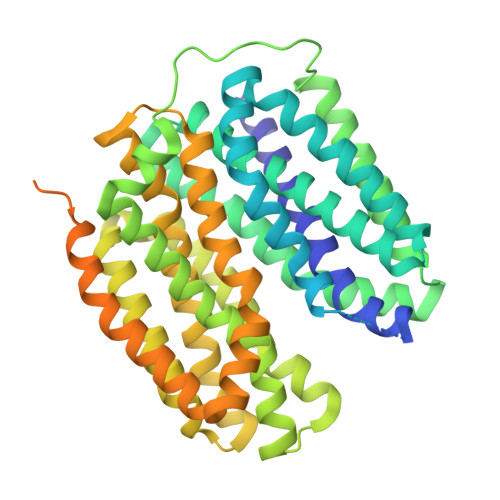 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q05940 (Homo sapiens) Explore Q05940 Go to UniProtKB: Q05940 | |||||
PHAROS: Q05940 GTEx: ENSG00000165646 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q05940 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| WRF (Subject of Investigation/LOI) Query on WRF | B [auth A] | 1-methyl-4-phenylpyridin-1-ium C12 H12 N FMGYKKMPNATWHP-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 31971134 |