Identification of Key Post‐modification Enzymes Involved in the Biosynthesis of Lanostane‐type Triterpenoids in the Medicinal Mushroom Antrodia camphorata
Zhang, Y.Q., Zhang, M., Wang, Z.L., Bao, Y.O., Wang, Y.Q., Tian, Y.G., Ye, L., Ye, M.(2025) Angew Chem Int Ed Engl 64: e202420104-e202420104
- PubMed: 39617723
- DOI: https://doi.org/10.1002/anie.202420104
- Primary Citation of Related Structures:
8WZZ - PubMed Abstract:
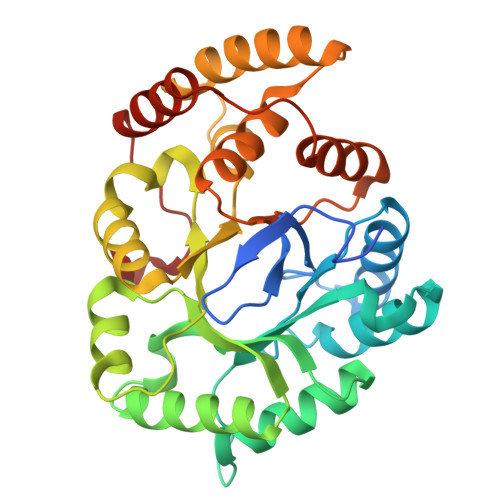
Through different gene mining strategies, three key enzymes (AcCYP4, AcSDR6, AcSMT1) involved in the downstream biosynthesis of major lanostane-type triterpenoids were discovered and identified from Antrodia camphorata. The catalytic mechanisms of AcSDR6 were elucidated by crystal structure analysis. These post-modification enzymes could be used to synthesize at least 11 major Antrodia lanostanoids.
- State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing, 100191, China.
Organizational Affiliation: