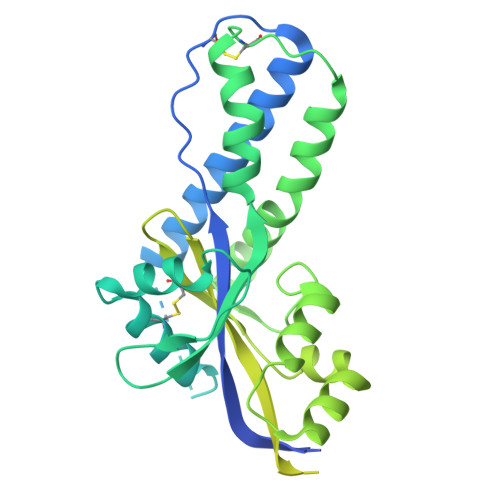
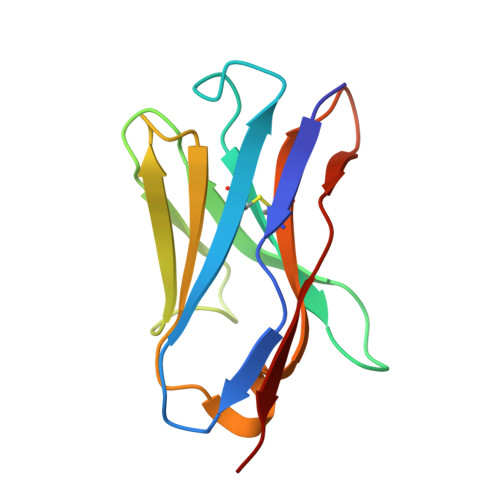
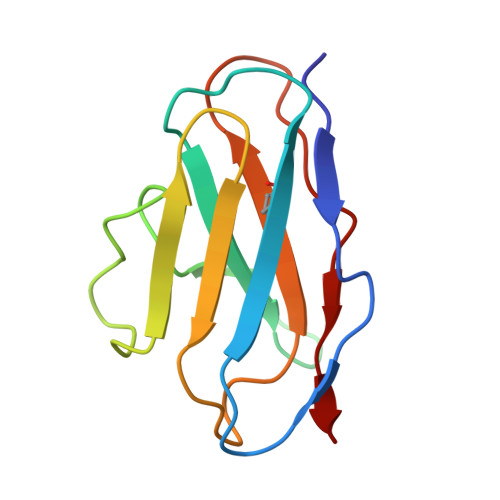
A potent broad-spectrum neutralizing antibody targeting a conserved region of the prefusion RSV F protein.
Sun, Y., Liu, L., Qiang, H., Sun, H., Jiang, Y., Ren, L., Jiang, Z., Lei, S., Chen, L., Wang, Y., Lin, X., Wang, G., Huang, Y., Fu, Y., Shi, Y., Chen, X., Yu, H., Li, S., Luo, W., Liu, E., Zheng, Q., Zheng, Z., Xia, N.(2024) Nat Commun 15: 10085-10085
- PubMed: 39572535
- DOI: https://doi.org/10.1038/s41467-024-54384-x
- Primary Citation of Related Structures:
8WZ3, 8WZ4, 8WZ5, 8WZE - PubMed Abstract:
Respiratory syncytial virus (RSV) poses a significant public health challenge, especially among children. Although palivizumab and nirsevimab, neutralizing antibodies (nAbs) targeting the RSV F protein, have been used for prophylaxis, their limitations underscore the need for more effective alternatives. Herein, we present a potent and broad nAb, named 5B11, which exhibits nanogram level of unbiased neutralizing activities against both RSV-A and -B subgroups. Notably, 5B11 shows a ~20-fold increase in neutralizing efficacy compared to 1129 (the murine precursor of palivizumab) and approximately a 3-fold increase in neutralizing efficacy against B18537 in comparison to nirsevimab. Cryo-electron microscopy analysis reveals 5B11's mechanism of action by targeting a highly conserved epitope within site V, offering a promising strategy with potentially lower risk of escape mutants. Antiviral testing in a female cotton rat model demonstrated that low-dose (1.5 mg/kg) administration of 5B11 achieved comparable prophylactic efficacy to that achieved by high-dose (15 mg/kg) of 1129. Furthermore, the humanized 5B11 showed a superior in vivo antiviral activity against B18537 infection compared to nirsevimab and palivizumab. Therefore, 5B11 is a promising RSV prophylactic candidate applicable to broad prevention of RSV infection.
- State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, P. R. China.
Organizational Affiliation: