Human AGT1-rBAT complex in the apo-state
Yan, R.H., Shi, T.H., Dong, J.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Amino acid transporter heavy chain SLC3A1 | A [auth C], C [auth D] | 736 | Homo sapiens | Mutation(s): 0 Gene Names: SLC3A1, NBAT Membrane Entity: Yes | 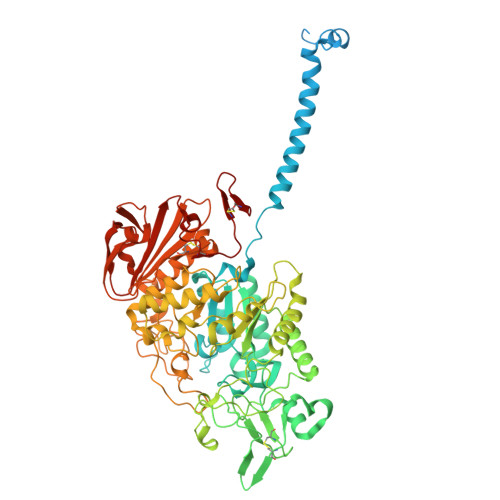 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q07837 (Homo sapiens) Explore Q07837 Go to UniProtKB: Q07837 | |||||
PHAROS: Q07837 GTEx: ENSG00000138079 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q07837 | ||||
Glycosylation | |||||
| Glycosylation Sites: 5 | Go to GlyGen: Q07837-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Solute carrier family 7 member 13 | B, D [auth A] | 498 | Homo sapiens | Mutation(s): 0 Gene Names: SLC7A13, AGT1, XAT2 Membrane Entity: Yes | 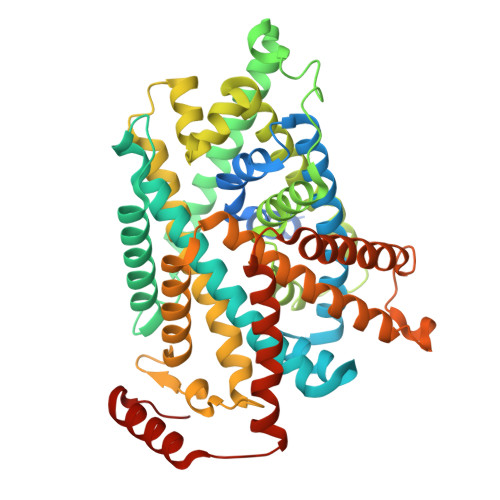 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q8TCU3 (Homo sapiens) Explore Q8TCU3 Go to UniProtKB: Q8TCU3 | |||||
GTEx: ENSG00000164893 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8TCU3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CA Query on CA | O [auth C], P [auth D] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | -- |