Cryo-EM structure of DSR2-TTP
Zhang, H., Li, Z., Li, X.Z.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| SIR2-like domain-containing protein | 1,005 | Bacillus subtilis | Mutation(s): 0 Gene Names: B4122_2870 EC: 3.2.2.5 | 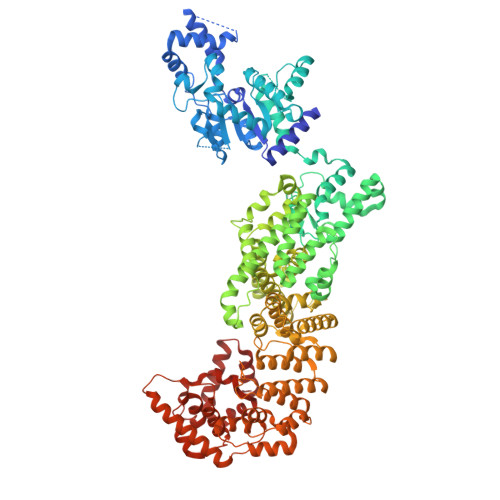 | |
UniProt | |||||
Find proteins for P0DXN8 (Bacillus subtilis) Explore P0DXN8 Go to UniProtKB: P0DXN8 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0DXN8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| tail tube protein(TTP) | 264 | Bacillus subtilis | Mutation(s): 0 Gene Names: B4122_1986 | 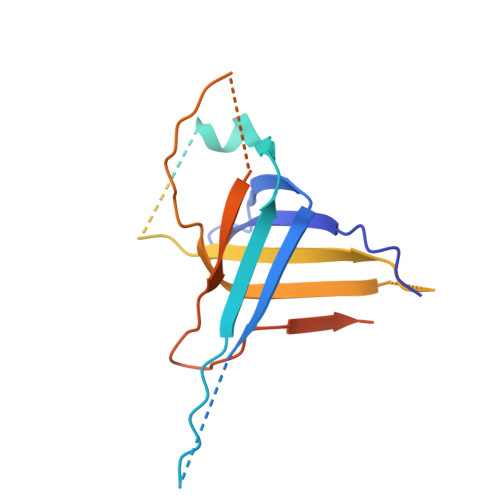 | |
UniProt | |||||
Find proteins for A0AAE9G628 (Bacillus phage SPR) Explore A0AAE9G628 Go to UniProtKB: A0AAE9G628 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0AAE9G628 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Other government | -- |