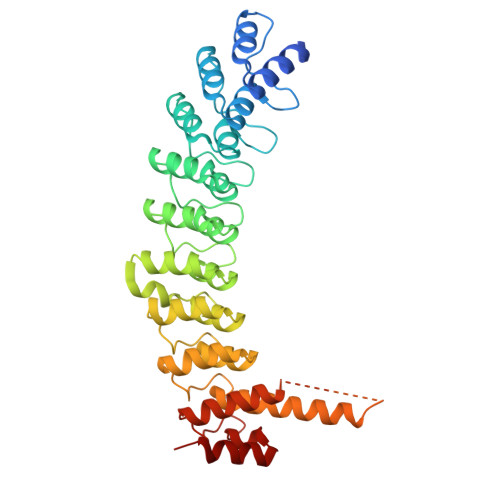
Structural requirements for activity of Mind bomb1 in Notch signaling.
Cao, R., Gozlan, O., Airich, A., Tveriakhina, L., Zhou, H., Jiang, H., Cole, P.A., Aster, J.C., Klein, T., Sprinzak, D., Blacklow, S.C.(2024) Structure 32: 1667-1676.e5
- PubMed: 39121852
- DOI: https://doi.org/10.1016/j.str.2024.07.011
- Primary Citation of Related Structures:
8V0D, 8V0E - PubMed Abstract:
Mind bomb 1 (MIB1) is a RING E3 ligase that ubiquitinates Notch ligands, a necessary step for induction of Notch signaling. The structural basis for binding of the JAG1 ligand by the N-terminal region of MIB1 is known, yet how the ankyrin (ANK) and RING domains of MIB1 cooperate to catalyze ubiquitin transfer from E2∼Ub to Notch ligands remains unclear. Here, we show that the third RING domain and adjacent coiled coil region (ccRING3) drive MIB1 dimerization and that MIB1 ubiquitin transfer activity relies solely on ccRING3. We report X-ray crystal structures of a UbcH5B-ccRING3 complex and the ANK domain. Directly tethering the MIB1 N-terminal region to ccRING3 forms a minimal MIB1 protein sufficient to induce a Notch response in receiver cells and rescue mib knockout phenotypes in flies. Together, these studies define the functional elements of an E3 ligase needed for ligands to induce a Notch signaling response.
- Department of Biological Chemistry and Molecular Pharmacology, Blavatnik Institute, Harvard Medical School, Boston, MA 02115, USA.
Organizational Affiliation: