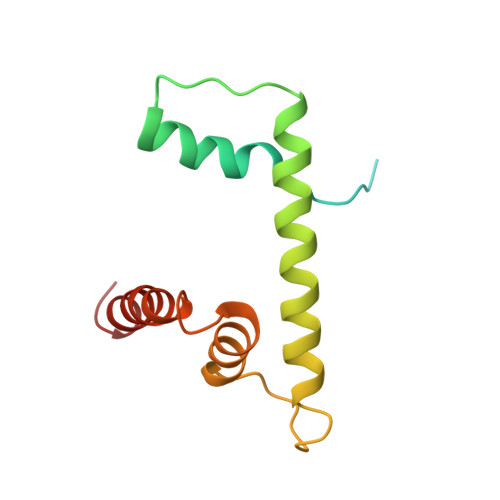
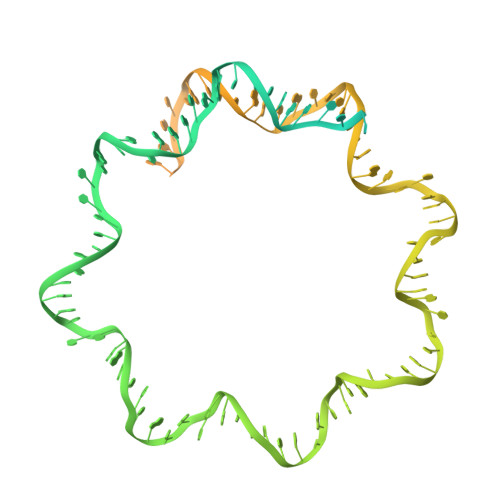
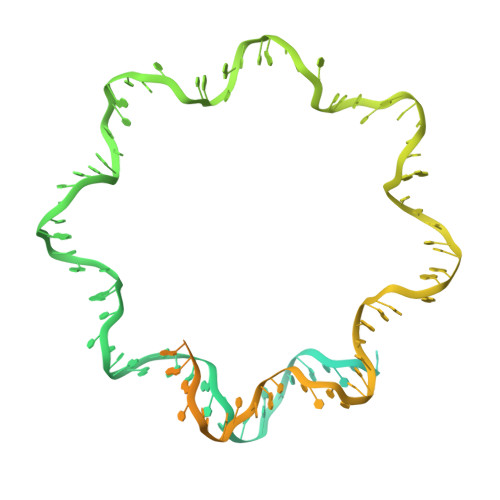
Structural mechanism of HP1⍺-dependent transcriptional repression and chromatin compaction.
Sokolova, V., Miratsky, J., Svetlov, V., Brenowitz, M., Vant, J., Lewis, T.S., Dryden, K., Lee, G., Sarkar, S., Nudler, E., Singharoy, A., Tan, D.(2024) Structure 32: 2094-2106.e6
- PubMed: 39383876
- DOI: https://doi.org/10.1016/j.str.2024.09.013
- Primary Citation of Related Structures:
8UXQ - PubMed Abstract:
Heterochromatin protein 1 (HP1) plays a central role in establishing and maintaining constitutive heterochromatin. However, the mechanisms underlying HP1-nucleosome interactions and their contributions to heterochromatin functions remain elusive. Here, we present the cryoelectron microscopy (cryo-EM) structure of an HP1α dimer bound to an H2A.Z-nucleosome, revealing two distinct HP1α-nucleosome interfaces. The primary HP1α binding site is located at the N terminus of histone H3, specifically at the trimethylated lysine 9 (K9me3) region, while a secondary binding site is situated near histone H2B, close to nucleosome superhelical location 4 (SHL4). Our biochemical data further demonstrates that HP1α binding influences the dynamics of DNA on the nucleosome. It promotes DNA unwrapping near the nucleosome entry and exit sites while concurrently restricting DNA accessibility in the vicinity of SHL4. Our study offers a model for HP1α-mediated heterochromatin maintenance and gene silencing. It also sheds light on the H3K9me-independent role of HP1 in responding to DNA damage.
- Department of Pharmacological Sciences, Stony Brook University, Stony Brook, NY, USA.
Organizational Affiliation: