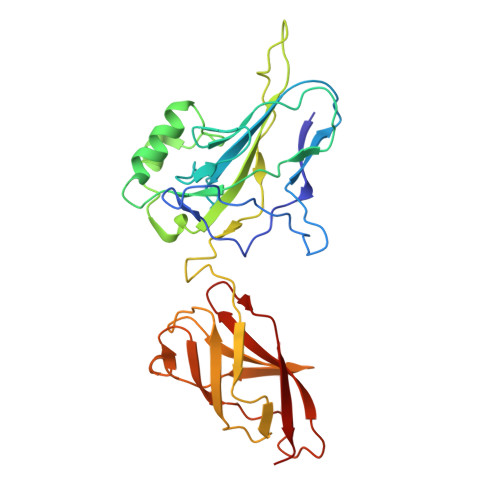
Stepwise neofunctionalization of the NF-kappa B family member Rel during vertebrate evolution.
Daly, A.E., Chang, A.B., Purbey, P.K., Williams, K.J., Li, S., Redelings, B.D., Yeh, G., Wu, Y., Pope, S.D., Venkatesh, B., Li, S., Nguyen, K., Rodrigues, J., Jorgensen, K., Dasgupta, A., Siggers, T., Chen, L., Smale, S.T.(2025) Nat Immunol 26: 760-774
- PubMed: 40307452
- DOI: https://doi.org/10.1038/s41590-025-02138-2
- Primary Citation of Related Structures:
8U9L - PubMed Abstract:
Adaptive immunity and the five vertebrate NF-κB family members first emerged in cartilaginous fish, suggesting that NF-κB family divergence helped to facilitate adaptive immunity. One specialized function of the NF-κB Rel protein in macrophages is activation of Il12b, which encodes a key regulator of T cell development. We found that Il12b exhibits much greater Rel dependence than inducible innate immunity genes in macrophages, with the unique function of Rel dimers depending on a heightened intrinsic DNA-binding affinity. Chromatin immunoprecipitation followed by sequencing experiments defined differential DNA-binding preferences of NF-κB family members genome-wide, and X-ray crystallography revealed a key residue that supports the heightened DNA-binding affinity of Rel dimers. Unexpectedly, this residue, the heightened affinity of Rel dimers, and the portion of the Il12b promoter bound by Rel dimers were largely restricted to mammals. Our findings reveal major structural transitions in an NF-κB family member and one of its key target promoters at a late stage of vertebrate evolution that apparently contributed to immunoregulatory rewiring in mammalian species.
- Department of Microbiology, Immunology, and Molecular Genetics, UCLA, Los Angeles, CA, USA.
Organizational Affiliation: