Site-Specific Arrangement and Structure Determination of Minor Groove Binding Molecules in Self-Assembled Three-Dimensional DNA Crystals.
Simmons, C.R., Buchberger, A., Henry, S.J.W., Novacek, A., Fahmi, N.E., MacCulloch, T., Stephanopoulos, N., Yan, H.(2023) J Am Chem Soc 145: 26075-26085
- PubMed: 37987645
- DOI: https://doi.org/10.1021/jacs.3c07802
- Primary Citation of Related Structures:
8T7B, 8T7X, 8T80, 8TA8, 8TA9, 8TAJ, 8TAM, 8TAP, 8TAQ, 8TB3, 8TB4, 8TB8, 8TBD, 8TBO, 8TC2, 8TC4, 8TC6, 8TDT - PubMed Abstract:
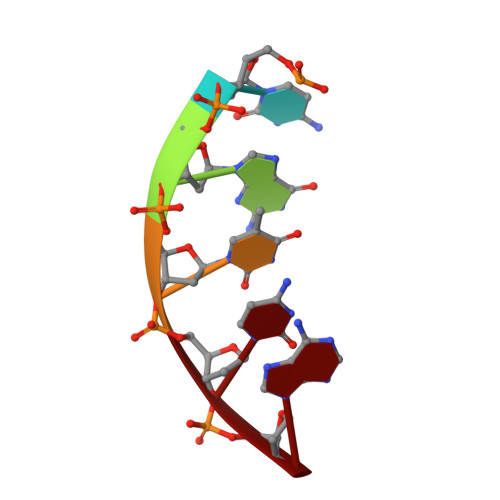
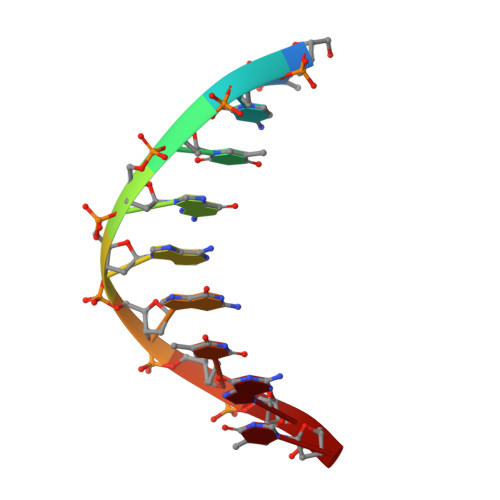
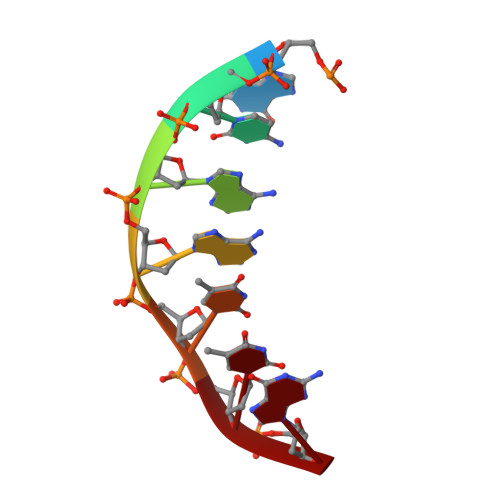
The structural analysis of guest molecules in rationally designed and self-assembling DNA crystals has proven an elusive goal since its conception. Oligonucleotide frameworks provide an especially attractive route toward studying DNA-binding molecules by using three-dimensional lattices with defined sequence and structure. In this work, we site-specifically position a suite of minor groove binding molecules, and solve their structures via X-ray crystallography as a proof-of-principle toward scaffolding larger guest species. Two crystal motifs were used to precisely immobilize the molecules DAPI, Hoechst, and netropsin at defined positions in the lattice, allowing us to control occupancy within the crystal. We also solved the structure of a three-ring imidazole-pyrrole-pyrrole polyamide molecule, which sequence-specifically packs in an antiparallel dimeric arrangement within the minor groove. Finally, we engineered a crystal designed to position both netropsin and the polyamide at two distinct locations within the same lattice. Our work elucidates the design principles for the spatial arrangement of functional guests within lattices and opens new potential opportunities for the use of DNA crystals to display and structurally characterize small molecules, peptides, and ultimately proteins of unknown structure.
- Biodesign Center for Molecular Design and Biomimetics, Arizona State University 1001 S. McAllister Ave., Tempe, Arizona 85287, United States.
Organizational Affiliation: