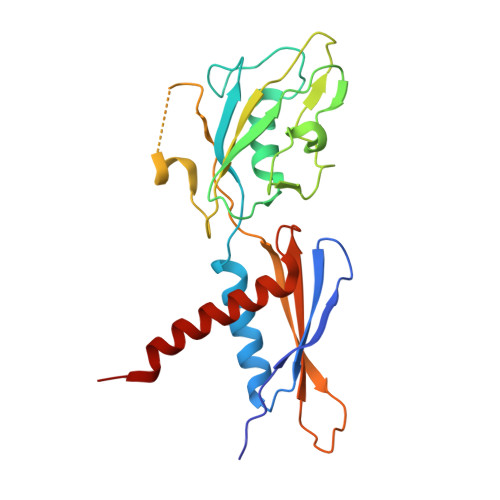
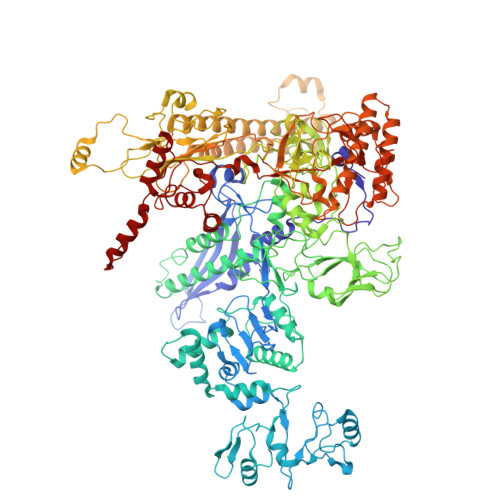
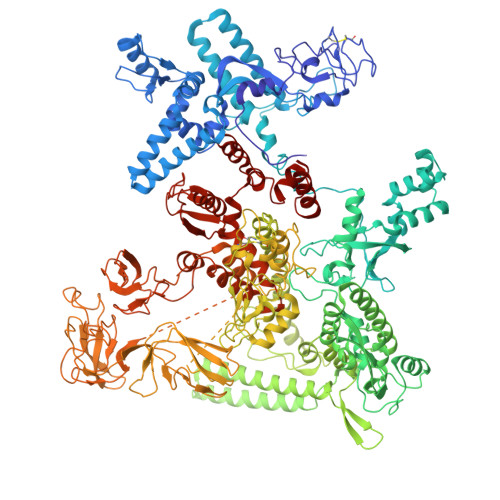
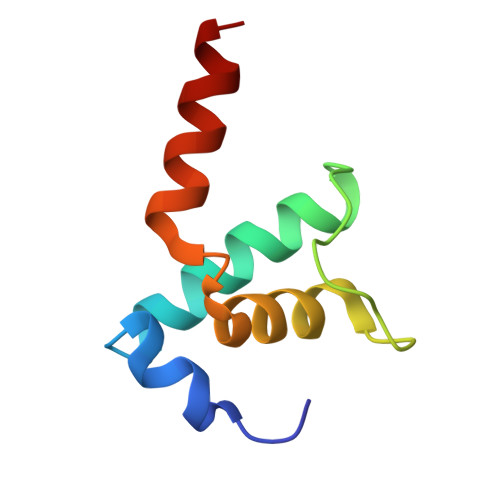
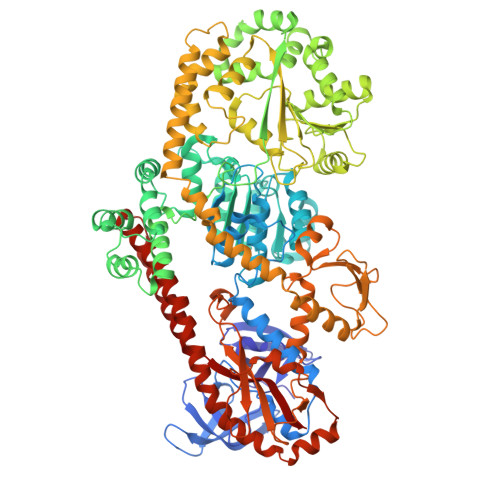
RapA opens the RNA polymerase clamp to disrupt post-termination complexes and prevent cytotoxic R-loop formation.
Brewer, J.J., Inlow, K., Mooney, R.A., Bosch, B., Olinares, P.D.B., Marcelino, L.P., Chait, B.T., Landick, R., Gelles, J., Campbell, E.A., Darst, S.A.(2025) Nat Struct Mol Biol 32: 639-649
- PubMed: 39779919
- DOI: https://doi.org/10.1038/s41594-024-01447-8
- Primary Citation of Related Structures:
8SZW, 8T00, 8T02, 8T0L - PubMed Abstract:
Following transcript release during intrinsic termination, Escherichia coli RNA polymerase (RNAP) often remains associated with DNA in a post-termination complex (PTC). RNAPs in PTCs are removed from the DNA by the SWI2/SNF2 adenosine triphosphatase (ATPase) RapA. Here we determined PTC structures on negatively supercoiled DNA and with RapA engaged to dislodge the PTC. We found that core RNAP in the PTC can unwind DNA and initiate RNA synthesis but is prone to producing R-loops. Nucleotide binding to RapA triggers a conformational change that opens the RNAP clamp, allowing DNA in the RNAP cleft to reanneal and dissociate. We show that RapA helps to control cytotoxic R-loop formation in vivo, likely by disrupting PTCs. We suggest that analogous ATPases acting on PTCs to suppress transcriptional noise and R-loop formation may be widespread. These results hold importance for the bacterial transcription cycle and highlight a role for RapA in maintaining genome stability.
- Laboratory of Molecular Biophysics, The Rockefeller University, New York, NY, USA.
Organizational Affiliation: