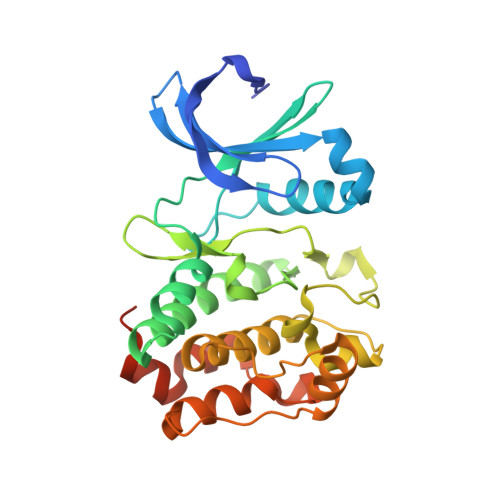
A biophysical framework for double-drugging kinases.
Kim, C., Ludewig, H., Hadzipasic, A., Kutter, S., Nguyen, V., Kern, D.(2023) Proc Natl Acad Sci U S A 120: e2304611120-e2304611120
- PubMed: 37590418
- DOI: https://doi.org/10.1073/pnas.2304611120
- Primary Citation of Related Structures:
8SSN, 8SSO, 8SSP - PubMed Abstract:
Selective orthosteric inhibition of kinases has been challenging due to the conserved active site architecture of kinases and emergence of resistance mutants. Simultaneous inhibition of distant orthosteric and allosteric sites, which we refer to as "double-drugging", has recently been shown to be effective in overcoming drug resistance. However, detailed biophysical characterization of the cooperative nature between orthosteric and allosteric modulators has not been undertaken. Here, we provide a quantitative framework for double-drugging of kinases employing isothermal titration calorimetry, Förster resonance energy transfer, coupled-enzyme assays, and X-ray crystallography. We discern positive and negative cooperativity for Aurora A kinase (AurA) and Abelson kinase (Abl) with different combinations of orthosteric and allosteric modulators. We find that a conformational equilibrium shift is the main principle governing cooperativity. Notably, for both kinases, we find a synergistic decrease of the required orthosteric and allosteric drug dosages when used in combination to inhibit kinase activities to clinically relevant inhibition levels. X-ray crystal structures of the double-drugged kinase complexes reveal the molecular principles underlying the cooperative nature of double-drugging AurA and Abl with orthosteric and allosteric inhibitors. Finally, we observe a fully closed conformation of Abl when bound to a pair of positively cooperative orthosteric and allosteric modulators, shedding light on the puzzling abnormality of previously solved closed Abl structures. Collectively, our data provide mechanistic and structural insights into rational design and evaluation of double-drugging strategies.
- Department of Biochemistry, Brandeis University, Waltham, MA 02454.
Organizational Affiliation: