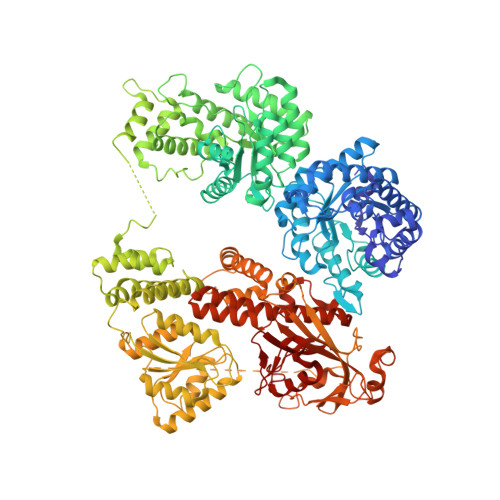
Structure of full-length cobalamin-dependent methionine synthase and cofactor loading captured in crystallo.
Mendoza, J., Purchal, M., Yamada, K., Koutmos, M.(2023) Nat Commun 14: 6365-6365
- PubMed: 37821448
- DOI: https://doi.org/10.1038/s41467-023-42037-4
- Primary Citation of Related Structures:
8SSC, 8SSD, 8SSE - PubMed Abstract:
Cobalamin-dependent methionine synthase (MS) is a key enzyme in methionine and folate one-carbon metabolism. MS is a large multi-domain protein capable of binding and activating three substrates: homocysteine, folate, and S-adenosylmethionine for methylation. Achieving three chemically distinct methylations necessitates significant domain rearrangements to facilitate substrate access to the cobalamin cofactor at the right time. The distinct conformations required for each reaction have eluded structural characterization as its inherently dynamic nature renders structural studies difficult. Here, we use a thermophilic MS homolog (tMS) as a functional MS model. Its exceptional stability enabled characterization of MS in the absence of cobalamin, marking the only studies of a cobalamin-binding protein in its apoenzyme state. More importantly, we report the high-resolution full-length MS structure, ending a multi-decade quest. We also capture cobalamin loading in crystallo, providing structural insights into holoenzyme formation. Our work paves the way for unraveling how MS orchestrates large-scale domain rearrangements crucial for achieving challenging chemistries.
- Department of Chemistry, University of Michigan, Ann Arbor, MI, 48109, USA.
Organizational Affiliation: