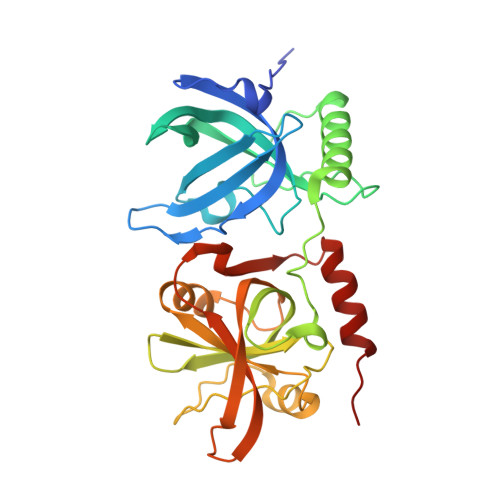
Human POT1 protects the telomeric ds-ss DNA junction by capping the 5' end of the chromosome.
Tesmer, V.M., Brenner, K.A., Nandakumar, J.(2023) Science 381: 771-778
- PubMed: 37590346
- DOI: https://doi.org/10.1126/science.adi2436
- Primary Citation of Related Structures:
8SH0, 8SH1 - PubMed Abstract:
Protection of telomeres 1 (POT1) is the 3' single-stranded overhang-binding telomeric protein that prevents an ataxia telangiectasia and Rad3-related (ATR) DNA damage response (DDR) at chromosome ends. What precludes the DDR machinery from accessing the telomeric double-stranded-single-stranded junction is unknown. We demonstrate that human POT1 binds this junction by recognizing the phosphorylated 5' end of the chromosome. High-resolution crystallographic structures reveal that the junction is capped by POT1 through a "POT-hole" surface, the mutation of which compromises junction protection in vitro and telomeric 5'-end definition and DDR suppression in human cells. Whereas both mouse POT1 paralogs bind the single-stranded overhang, POT1a, not POT1b, contains a POT-hole and binds the junction, which explains POT1a's sufficiency for end protection. Our study shifts the paradigm for DDR suppression at telomeres by highlighting the importance of protecting the double-stranded-single-stranded junction.
- Department of Molecular, Cellular and Developmental Biology, University of Michigan, Ann Arbor, MI 48109, USA.
Organizational Affiliation: