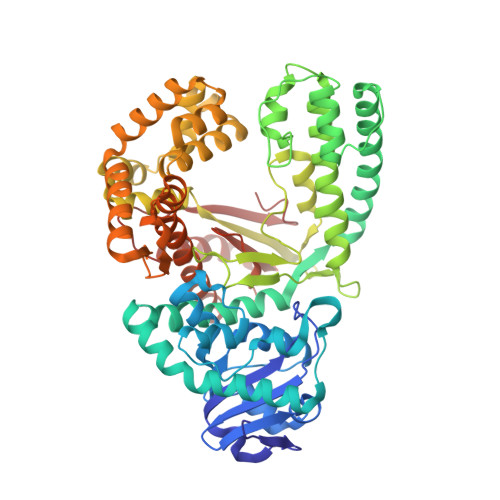
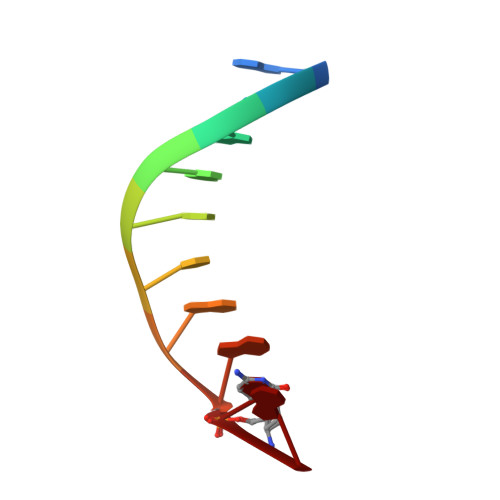
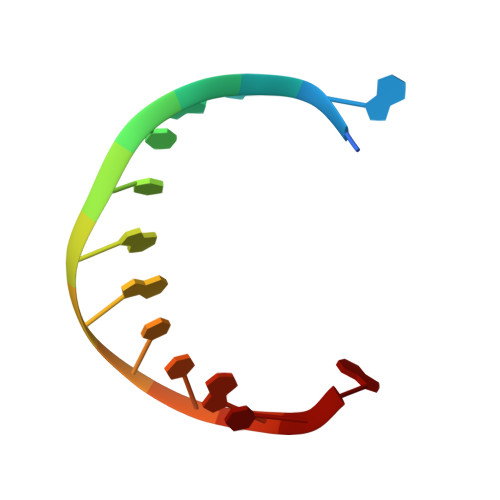
Trivalent rare earth metal cofactors confer rapid NP-DNA polymerase activity.
Lelyveld, V.S., Fang, Z., Szostak, J.W.(2023) Science 382: 423-429
- PubMed: 37883544
- DOI: https://doi.org/10.1126/science.adh5339
- Primary Citation of Related Structures:
8SCG, 8SCI, 8SCJ, 8SCK, 8SCL, 8SCM, 8SCN, 8SCO, 8SCP, 8SCQ, 8SCR, 8SCS, 8SCT, 8SCU - PubMed Abstract:
A DNA polymerase with a single mutation and a divalent calcium cofactor catalyzes the synthesis of unnatural N3'→P5' phosphoramidate (NP) bonds to form NP-DNA. However, this template-directed phosphoryl transfer activity remains orders of magnitude slower than native phosphodiester synthesis. Here, we used time-resolved x-ray crystallography to show that NP-DNA synthesis proceeds with a single detectable calcium ion in the active site. Using insights from isotopic and elemental effects, we propose that one-metal-ion electrophilic substrate activation is inferior to the native two-metal-ion mechanism. We found that this deficiency in divalent activation could be ameliorated by trivalent rare earth and post-transition metal cations, substantially enhancing NP-DNA synthesis. Scandium(III), in particular, confers highly specific NP activity with kinetics enhanced by more than 100-fold over calcium(II), yielding NP-DNA strands up to 100 nucleotides in length.
- Center for Computational and Integrative Biology, Department of Molecular Biology, Massachusetts General Hospital, Boston, MA 02114, USA.
Organizational Affiliation: