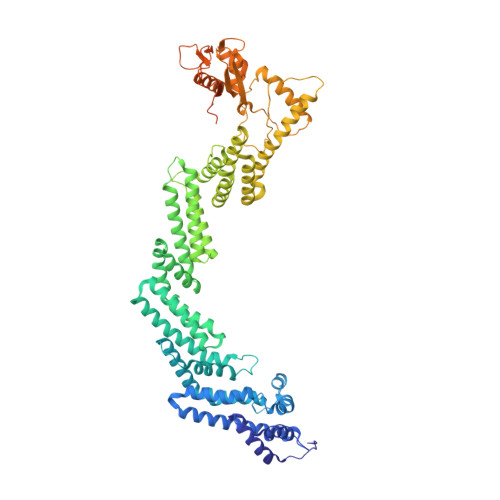
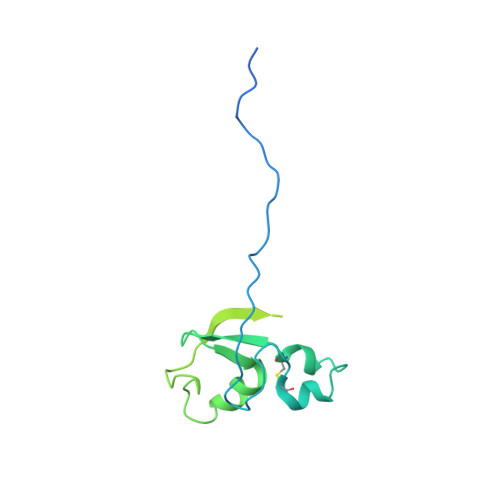
Mechanism of degrader-targeted protein ubiquitinability.
Crowe, C., Nakasone, M.A., Chandler, S., Craigon, C., Sathe, G., Tatham, M.H., Makukhin, N., Hay, R.T., Ciulli, A.(2024) Sci Adv 10: eado6492-eado6492
- PubMed: 39392888
- DOI: https://doi.org/10.1126/sciadv.ado6492
- Primary Citation of Related Structures:
8RWZ, 8RX0 - PubMed Abstract:
Small-molecule degraders of disease-driving proteins offer a clinically proven modality with enhanced therapeutic efficacy and potential to tackle previously undrugged targets. Stable and long-lived degrader-mediated ternary complexes drive fast and profound target degradation; however, the mechanisms by which they affect target ubiquitination remain elusive. Here, we show cryo-EM structures of the VHL Cullin 2 RING E3 ligase with the degrader MZ1 directing target protein Brd4 BD2 toward UBE2R1-ubiquitin, and Lys 456 at optimal positioning for nucleophilic attack. In vitro ubiquitination and mass spectrometry illuminate a patch of favorably ubiquitinable lysines on one face of Brd4 BD2 , with cellular degradation and ubiquitinomics confirming the importance of Lys 456 and nearby Lys 368 /Lys 445 , identifying the "ubiquitination zone." Our results demonstrate the proficiency of MZ1 in positioning the substrate for catalysis, the favorability of Brd4 BD2 for ubiquitination by UBE2R1, and the flexibility of CRL2 for capturing suboptimal lysines. We propose a model for ubiquitinability of degrader-recruited targets, providing a mechanistic blueprint for further rational drug design.
- Centre for Targeted Protein Degradation, School of Life Sciences, University of Dundee, 1 James Lindsay Place, Dundee DD1 5JJ, UK.
Organizational Affiliation: