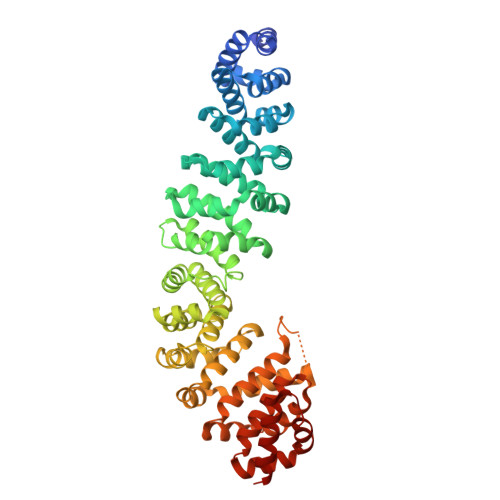
Structure-Based Design of Bicyclic Helical Peptides That Target the Oncogene beta-Catenin.
Yeste-Vazquez, A., Paulussen, F.M., Wendt, M., Klintrot, R., Schulte, C., Wallraven, K., van Gijzel, L., Simeonov, B., van der Gaag, M., Gerber, A., Maric, H.M., Hennig, S., Grossmann, T.N.(2024) Angew Chem Int Ed Engl 63: e202411749-e202411749
- PubMed: 39167026
- DOI: https://doi.org/10.1002/anie.202411749
- Primary Citation of Related Structures:
8RU3, 8RU4 - PubMed Abstract:
The inhibition of intracellular protein-protein interactions is challenging, in particular, when involved interfaces lack pronounced cavities. The transcriptional co-activator protein and oncogene β‑catenin is a prime example of such a challenging target. Despite extensive targeting efforts, available high-affinity binders comprise only large molecular weight Inhibitors. This hampers the further development of therapeutically useful compounds. Herein, we report the design of a considerably smaller peptidomimetic scaffold derived from the α-helical β‑catenin-binding motif of Axin. Sequence maturation and bicyclization provided a stitched peptide with an unprecedented crosslink architecture. The binding mode and site were confirmed by a crystal structure. Further derivatization yielded a β-catenin inhibitor with single-digit micromolar activity in a cell-based assay. This study sheds a light on how to design helix mimetics with reduced molecular weight thereby improving their biological activity.
- VU Amsterdam, Chemisty and Pharmaceutical Sciences, NETHERLANDS.
Organizational Affiliation: