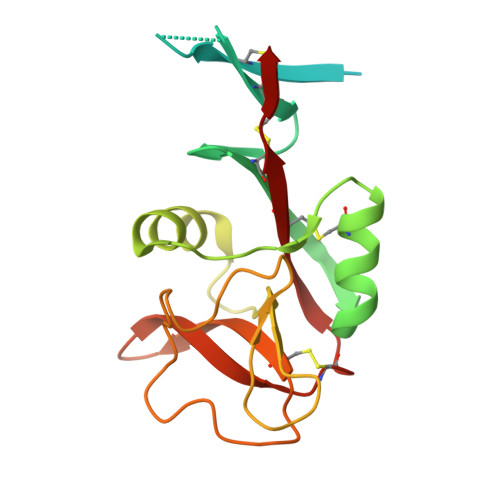
Interactions that define the arrangement of sugar-binding sites in BDCA-2 and dectin-2 dimers.
Liu, Y., Kim, J.W., Feinberg, H., Cull, N., Weis, W.I., Taylor, M.E., Drickamer, K.(2024) Glycobiology 34
- PubMed: 39361900
- DOI: https://doi.org/10.1093/glycob/cwae082
- Primary Citation of Related Structures:
8ROV - PubMed Abstract:
The sugar-binding receptors dectin-2 and blood dendritic cell antigen 2 (BDCA-2) bind oligosaccharide ligands through extracellular carbohydrate-recognition domains (CRDs) and initiate intracellular signaling through Fc receptor γ adapters (FcRγ). Dectin-2 stimulates macrophages in response to pathogen binding while BDCA-2 modulates cytokine production in plasmacytoid dendritic cells. The oligomeric states of these receptors and the orientations of their CRDs have been investigated by analysis of a naturally occurring disulfide-bonded variant of BDCA-2 and by replacement of transmembrane domains with N-terminal dimerization domains to create extracellular domain dimers of both dectin-2 and BDCA-2. Analysis of these constructs, as well as previously described crystal structures of the CRDs from these proteins and a novel structure of an extended version of the extracellular domain of dectin-2, showed that there is only limited interaction of the CRDs in the dimers, but interactions can be stabilized by the presence of the neck region. The resulting orientation of sugar-binding sites in the dimers would favor crosslinking of multiple dimers by oligosaccharide ligands, causing clustering of FcRγ to initiate signaling.
- Department of Life Sciences, Imperial College, London SW7 2AZ, United Kingdom.
Organizational Affiliation: