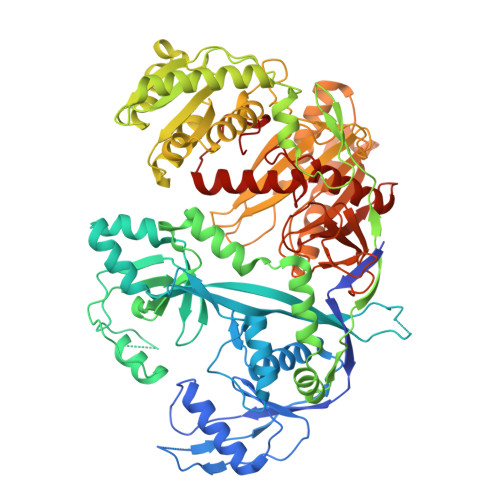
RNA-guided RNA silencing by an Asgard archaeal Argonaute.
Bastiaanssen, C., Bobadilla Ugarte, P., Kim, K., Finocchio, G., Feng, Y., Anzelon, T.A., Kostlbacher, S., Tamarit, D., Ettema, T.J.G., Jinek, M., MacRae, I.J., Joo, C., Swarts, D.C., Wu, F.(2024) Nat Commun 15: 5499-5499
- PubMed: 38951509
- DOI: https://doi.org/10.1038/s41467-024-49452-1
- Primary Citation of Related Structures:
8R3Z - PubMed Abstract:
Argonaute proteins are the central effectors of RNA-guided RNA silencing pathways in eukaryotes, playing crucial roles in gene repression and defense against viruses and transposons. Eukaryotic Argonautes are subdivided into two clades: AGOs generally facilitate miRNA- or siRNA-mediated silencing, while PIWIs generally facilitate piRNA-mediated silencing. It is currently unclear when and how Argonaute-based RNA silencing mechanisms arose and diverged during the emergence and early evolution of eukaryotes. Here, we show that in Asgard archaea, the closest prokaryotic relatives of eukaryotes, an evolutionary expansion of Argonaute proteins took place. In particular, a deep-branching PIWI protein (HrAgo1) encoded by the genome of the Lokiarchaeon 'Candidatus Harpocratesius repetitus' shares a common origin with eukaryotic PIWI proteins. Contrasting known prokaryotic Argonautes that use single-stranded DNA as guides and/or targets, HrAgo1 mediates RNA-guided RNA cleavage, and facilitates gene silencing when expressed in human cells and supplied with miRNA precursors. A cryo-EM structure of HrAgo1, combined with quantitative single-molecule experiments, reveals that the protein displays structural features and target-binding modes that are a mix of those of eukaryotic AGO and PIWI proteins. Thus, this deep-branching archaeal PIWI may have retained an ancestral molecular architecture that preceded the functional and mechanistic divergence of eukaryotic AGOs and PIWIs.
- Department of BioNanoScience, Kavli Institute of Nanoscience, Delft University of Technology, Delft, The Netherlands.
Organizational Affiliation: