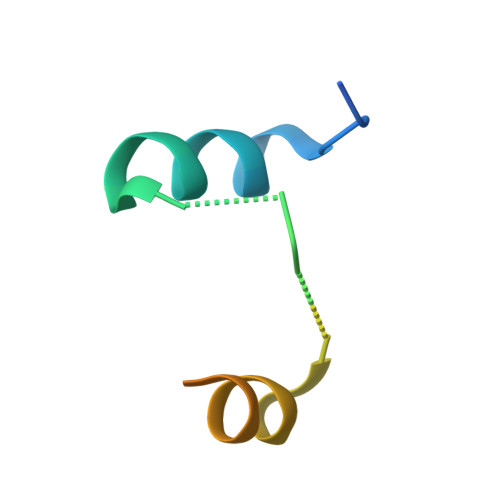
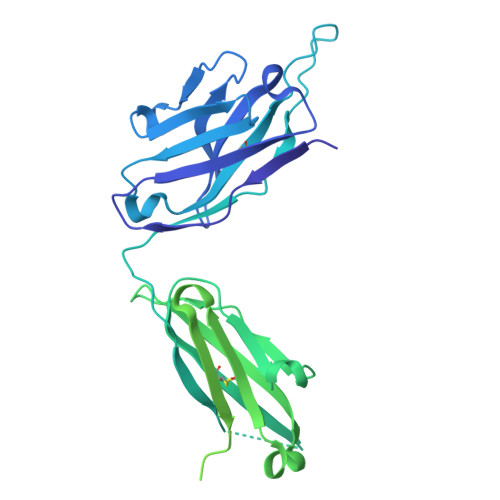
Epitope-focused immunogens targeting the hepatitis C virus glycoproteins induce broadly neutralizing antibodies.
Nagarathinam, K., Scheck, A., Labuhn, M., Stroh, L.J., Herold, E., Veselkova, B., Tune, S., Cramer, J.T., Rosset, S., Vollers, S.S., Bankwitz, D., Ballmaier, M., Boning, H., Roth, E., Khera, T., Ahsendorf-Abidi, H.P., Dittrich-Breiholz, O., Obleser, J., Nassal, M., Jack, H.M., Pietschmann, T., Correia, B.E., Krey, T.(2024) Sci Adv 10: eado2600-eado2600
- PubMed: 39642219
- DOI: https://doi.org/10.1126/sciadv.ado2600
- Primary Citation of Related Structures:
8QP6, 8QP7 - PubMed Abstract:
Hepatitis C virus (HCV) infection causes ~290,000 annual human deaths despite the highly effective antiviral treatment available. Several viral immune evasion mechanisms have hampered the development of an effective vaccine against HCV, among them the remarkable conformational flexibility within neutralization epitopes in the HCV antigens. Here, we report the design of epitope-focused immunogens displaying two distinct HCV cross-neutralization epitopes. We show that these immunogens induce a pronounced, broadly neutralizing antibody response in laboratory and transgenic human antibody mice. Monoclonal human antibodies isolated from immunized human antibody mice specifically recognized the grafted epitopes and neutralized four diverse HCV strains. Our results highlight a promising strategy for developing HCV immunogens and provide an encouraging paradigm for targeting structurally flexible epitopes to improve the induction of neutralizing antibodies.
- Institute of Biochemistry, Center of Structural and Cell Biology in Medicine, University of Lübeck, 23562 Lübeck, Germany.
Organizational Affiliation: