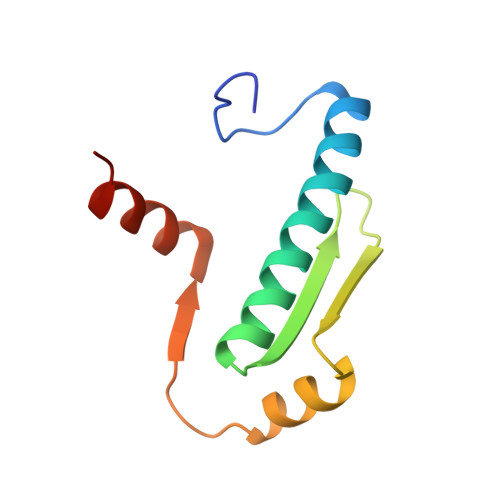
Folding of Class IIa HDAC Derived Peptides into alpha-helices Upon Binding to Myocyte Enhancer Factor-2 in Complex with DNA.
Chinellato, M., Perin, S., Carli, A., Lastella, L., Biondi, B., Borsato, G., Di Giorgio, E., Brancolini, C., Cendron, L., Angelini, A.(2024) J Mol Biology 436: 168541-168541
- PubMed: 38492719
- DOI: https://doi.org/10.1016/j.jmb.2024.168541
- Primary Citation of Related Structures:
8C84, 8PDE, 8Q9N, 8Q9P, 8Q9Q, 8Q9R - PubMed Abstract:
Interaction of transcription factor myocyte enhancer factor-2 (MEF2) family members with class IIa histone deacetylases (HDACs) has been implicated in a wide variety of diseases. Though considerable knowledge on this topic has been accumulated over the years, a high resolution and detailed analysis of the binding mode of multiple class IIa HDAC derived peptides with MEF2D is still lacking. To fulfil this gap, we report here the crystal structure of MEF2D in complex with double strand DNA and four different class IIa HDAC derived peptides, namely HDAC4, HDAC5, HDAC7 and HDAC9. All class IIa HDAC derived peptides form extended amphipathic α-helix structures that fit snugly in the hydrophobic groove of MEF2D domain. Binding mode of class IIa HDAC derived peptides to MEF2D is very similar and occur primarily through nonpolar interactions mediated by highly conserved branched hydrophobic amino acids. Further studies revealed that class IIa HDAC derived peptides are unstructured in solution and appear to adopt a folded α-helix structure only upon binding to MEF2D. Comparison of our peptide-protein complexes with previously characterized structures of MEF2 bound to different co-activators and co-repressors, highlighted both differences and similarities, and revealed the adaptability of MEF2 in protein-protein interactions. The elucidation of the three-dimensional structure of MEF2D in complex with multiple class IIa HDAC derived peptides provide not only a better understanding of the molecular basis of their interactions but also have implications for the development of novel antagonist.
- Department of Biology, University of Padua, Via U. Bassi 58, 35131 Padova, Italy.
Organizational Affiliation: