Cryo-EM structures of tau filaments from the brains of mice transgenic for human mutant P301S Tau.
Schweighauser, M., Murzin, A.G., Macdonald, J., Lavenir, I., Crowther, R.A., Scheres, S.H.W., Goedert, M.(2023) Acta Neuropathol Commun 11: 160-160
- PubMed: 37798679
- DOI: https://doi.org/10.1186/s40478-023-01658-y
- Primary Citation of Related Structures:
8Q92, 8Q96 - PubMed Abstract:
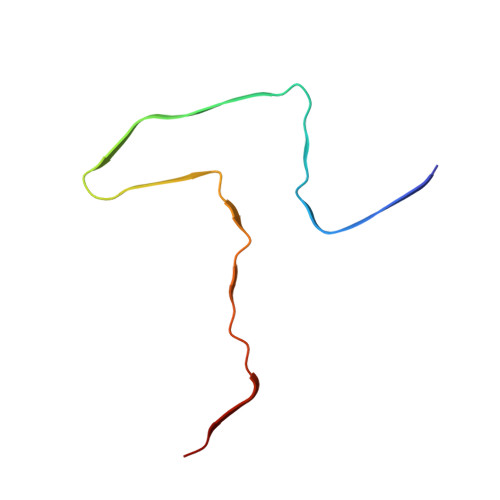
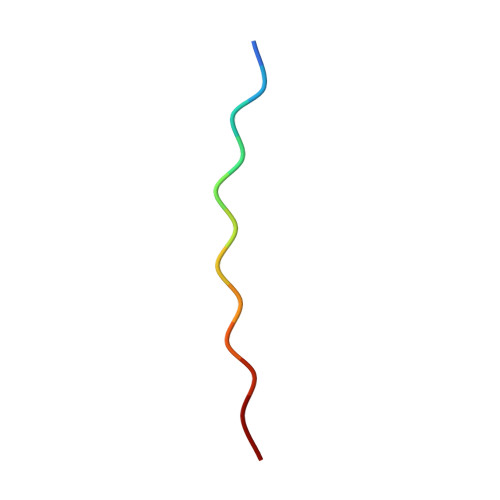
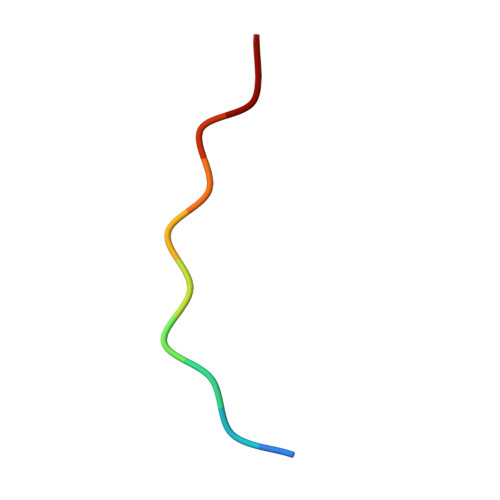
Mice transgenic for human mutant P301S tau are widely used as models for human tauopathies. They develop neurodegeneration and abundant filamentous inclusions made of human mutant four-repeat tau. Here we used electron cryo-microscopy (cryo-EM) to determine the structures of tau filaments from the brains of Tg2541 and PS19 mice. Both lines express human P301S tau (0N4R for Tg2541 and 1N4R for PS19) on mixed genetic backgrounds and downstream of different promoters (murine Thy1 for Tg2541 and murine Prnp for PS19). The structures of tau filaments from Tg2541 and PS19 mice differ from each other and those of wild-type tau filaments from human brains. The structures of tau filaments from the brains of humans with mutations P301L, P301S or P301T in MAPT are not known. Filaments from the brains of Tg2541 and PS19 mice share a substructure at the junction of repeats 2 and 3, which comprises residues I297-V312 of tau and includes the P301S mutation. The filament core from the brainstem of Tg2541 mice consists of residues K274-H329 of tau and two disconnected protein densities. Two non-proteinaceous densities are also in evidence. The filament core from the cerebral cortex of line PS19 extends from residues G271-P364 of tau. One strong non-proteinaceous density is also present. Unlike the tau filaments from human brains, the sequences following repeat 4 are missing from the cores of tau filaments from the brains of Tg2541 and PS19 mice.
- Medical Research Council Laboratory of Molecular Biology, Cambridge, UK.
Organizational Affiliation: