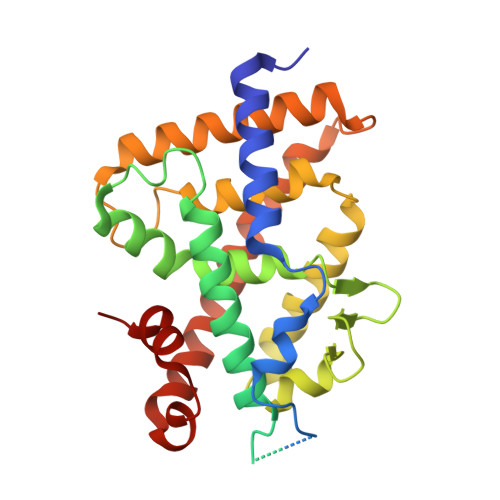
Design, synthesis, and biological activity of D-bishomo-1 alpha ,25-dihydroxyvitamin D 3 analogs and their crystal structures with the vitamin D nuclear receptor.
Fabisiak, A., Brzeminski, P., Sicinski, R.R., Rochel, N., Maj, E., Filip-Psurska, B., Wietrzyk, J., Plum, L.A., DeLuca, H.F.(2024) Eur J Med Chem 271: 116403-116403
- PubMed: 38615411
- DOI: https://doi.org/10.1016/j.ejmech.2024.116403
- Primary Citation of Related Structures:
8PZ6, 8PZ7, 8PZ8, 8PZ9, 8PZB - PubMed Abstract:
The biologically active metabolite of vitamin D 3 - calcitriol - is a hormone involved in the regulation of calcium-phosphate homeostasis, immunological processes and cell differentiation, being therefore essential for the proper functioning of the human body. This suggests many applications of this steroid in the treatment of diseases such as rickets, psoriasis and some cancers. Unfortunately, using therapeutic doses of calcitriol is associated with high concentrations of this compound which causes hypercalcemia. For this reason, new calcitriol analogs are constantly sought, devoid of calcemic effects but maintaining its beneficial properties. In this study, we present the synthesis of vitamin D derivatives characterized by an enlarged (seven-membered) ring D. Preparation of the designed vitamin D compounds required separate syntheses of crucial building blocks (C/D-rings fragments with side chain and rings A) which were combined by different methods, including Wittig-Horner reaction and Suzuki coupling. Biological activities of the target vitamin D analogs were assessed both in vitro and in vivo, demonstrating their significant potency compared to the natural hormone. Furthermore, the successful crystallization of these compounds with the vitamin D receptor (VDR) enabled us to investigate additional molecular interactions with this protein.
- Department of Chemistry, University of Warsaw, Pasteura 1, 02-093, Warsaw, Poland. Electronic address: afabisiak@chem.uw.edu.pl.
Organizational Affiliation: