Co-crystal structure of FKBP12, compound 7 and the FRB fragment of mTOR
Meyners, C., Deutscher, R.C.E., Hausch, F.(2023) ChemRxiv
Experimental Data Snapshot
Starting Model: experimental
View more details
(2023) ChemRxiv
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Peptidyl-prolyl cis-trans isomerase FKBP1A | 107 | Homo sapiens | Mutation(s): 1 Gene Names: FKBP1A, FKBP1, FKBP12 EC: 5.2.1.8 | 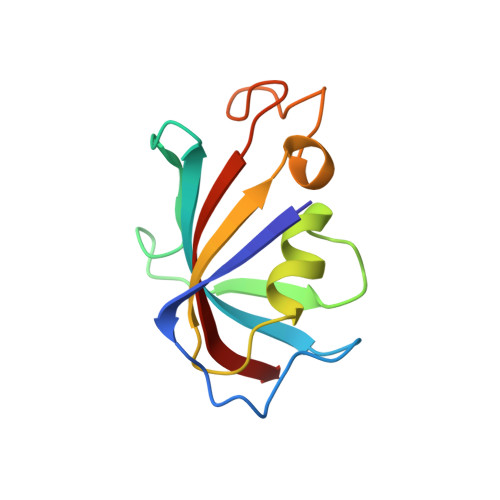 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P62942 (Homo sapiens) Explore P62942 Go to UniProtKB: P62942 | |||||
PHAROS: P62942 GTEx: ENSG00000088832 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P62942 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Serine/threonine-protein kinase mTOR | 98 | Homo sapiens | Mutation(s): 0 Gene Names: MTOR, FRAP, FRAP1, FRAP2, RAFT1, RAPT1 EC: 2.7.11.1 (PDB Primary Data), 2.7.10.2 (UniProt) | 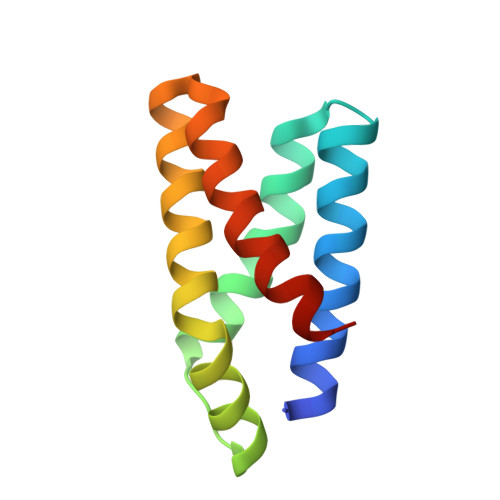 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P42345 (Homo sapiens) Explore P42345 Go to UniProtKB: P42345 | |||||
PHAROS: P42345 GTEx: ENSG00000198793 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P42345 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 0AN (Subject of Investigation/LOI) Query on 0AN | C [auth A] | (1~{S},5~{S},6~{R})-10-[3,5-bis(chloranyl)phenyl]sulfonyl-5-[(~{E})-2-(2-chlorophenyl)ethenyl]-3-(pyridin-2-ylmethyl)-3,10-diazabicyclo[4.3.1]decan-2-one C28 H26 Cl3 N3 O3 S LZVLUVFCVSNKDX-MGLWIZITSA-N |  | ||
| ACT Query on ACT | E [auth B] | ACETATE ION C2 H3 O2 QTBSBXVTEAMEQO-UHFFFAOYSA-M |  | ||
| CA Query on CA | D [auth A], F [auth B], G [auth B], H [auth B] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 39.912 | α = 90 |
| b = 64.68 | β = 90 |
| c = 93.349 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| German Federal Ministry for Education and Research | Germany | 03ZU1109EB |
| German Research Foundation (DFG) | Germany | HA5556 |