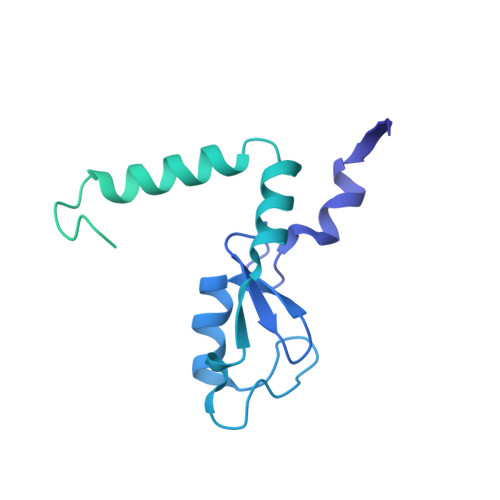
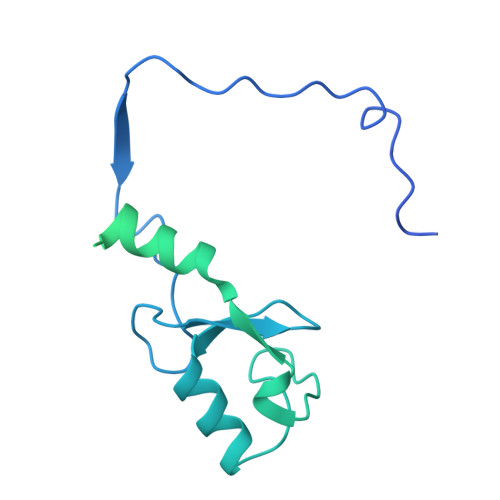
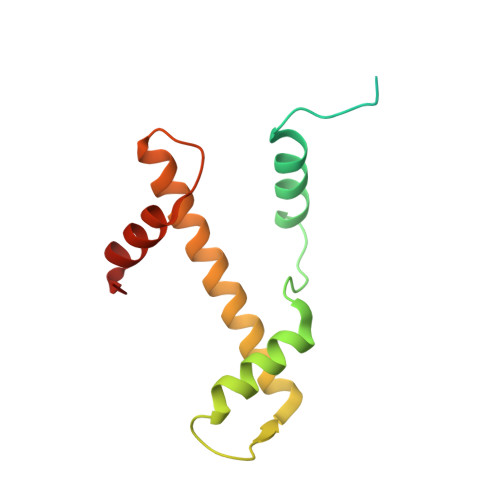
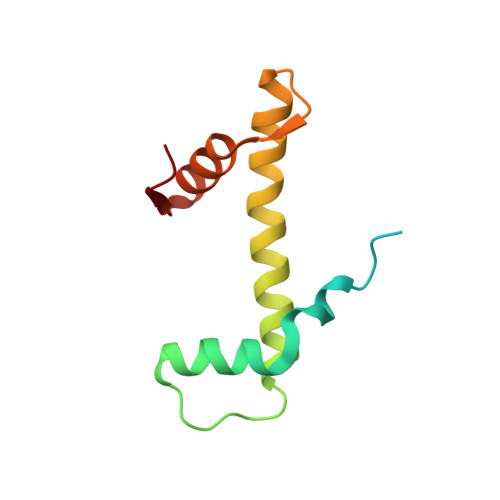
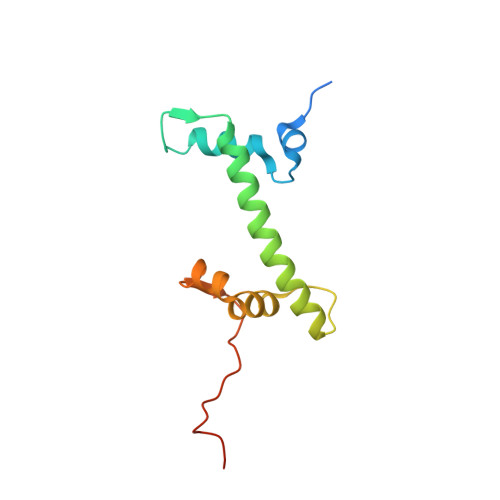
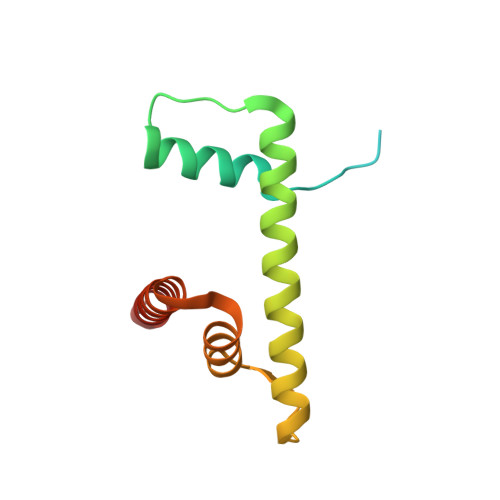
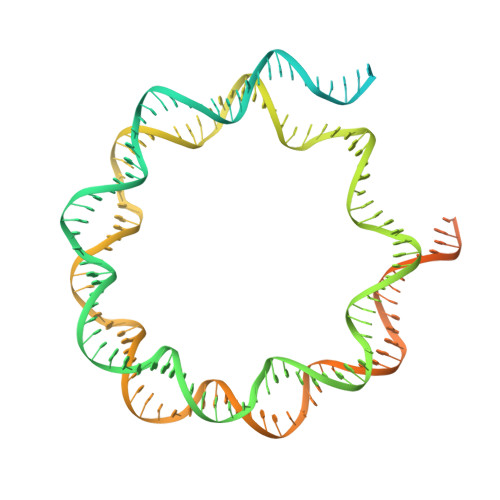
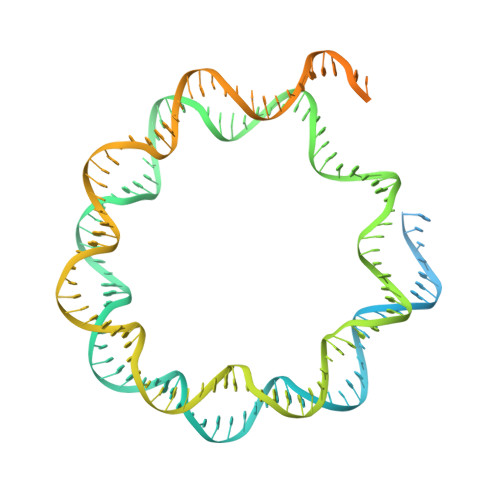
Structural basis of the histone ubiquitination read-write mechanism of RYBP-PRC1.
Ciapponi, M., Karlukova, E., Schkolziger, S., Benda, C., Muller, J.(2024) Nat Struct Mol Biol 31: 1023-1027
- PubMed: 38528151
- DOI: https://doi.org/10.1038/s41594-024-01258-x
- Primary Citation of Related Structures:
8PP6, 8PP7 - PubMed Abstract:
Histone H2A monoubiquitination (H2Aub1) by the PRC1 subunit RING1B entails a positive feedback loop, mediated by the RING1B-interacting protein RYBP. We uncover that human RYBP-PRC1 binds unmodified nucleosomes via RING1B but H2Aub1-modified nucleosomes via RYBP. RYBP interactions with both ubiquitin and the nucleosome acidic patch create the high binding affinity that favors RYBP- over RING1B-directed PRC1 binding to H2Aub1-modified nucleosomes; this enables RING1B to monoubiquitinate H2A in neighboring unmodified nucleosomes.
- Laboratory of Chromatin Biology, Max-Planck Institute of Biochemistry, Martinsried, Germany.
Organizational Affiliation: