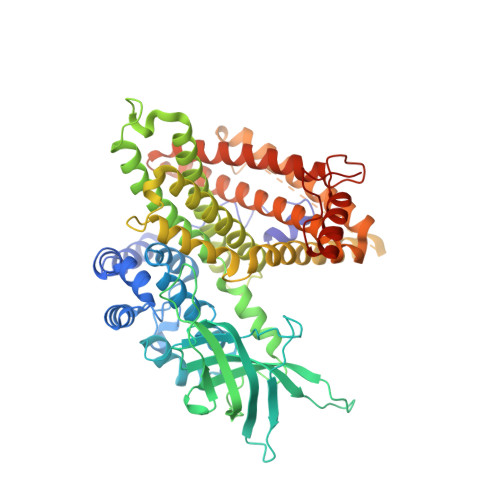
The assembly of the Mitochondrial Complex I Assembly complex uncovers a redox pathway coordination.
McGregor, L., Acajjaoui, S., Desfosses, A., Saidi, M., Bacia-Verloop, M., Schwarz, J.J., Juyoux, P., von Velsen, J., Bowler, M.W., McCarthy, A.A., Kandiah, E., Gutsche, I., Soler-Lopez, M.(2023) Nat Commun 14: 8248-8248
- PubMed: 38086790
- DOI: https://doi.org/10.1038/s41467-023-43865-0
- Primary Citation of Related Structures:
8PHE, 8PHF - PubMed Abstract:
The Mitochondrial Complex I Assembly (MCIA) complex is essential for the biogenesis of respiratory Complex I (CI), the first enzyme in the respiratory chain, which has been linked to Alzheimer's disease (AD) pathogenesis. However, how MCIA facilitates CI assembly, and how it is linked with AD pathogenesis, is poorly understood. Here we report the structural basis of the complex formation between the MCIA subunits ECSIT and ACAD9. ECSIT binding induces a major conformational change in the FAD-binding loop of ACAD9, releasing the FAD cofactor and converting ACAD9 from a fatty acid β-oxidation (FAO) enzyme to a CI assembly factor. We provide evidence that ECSIT phosphorylation downregulates its association with ACAD9 and is reduced in neuronal cells upon exposure to amyloid-β (Aβ) oligomers. These findings advance our understanding of the MCIA complex assembly and suggest a possible role for ECSIT in the reprogramming of bioenergetic pathways linked to Aβ toxicity, a hallmark of AD.
- Structural Biology Group, European Synchrotron Radiation Facility (ESRF), 38043, Grenoble, France.
Organizational Affiliation: