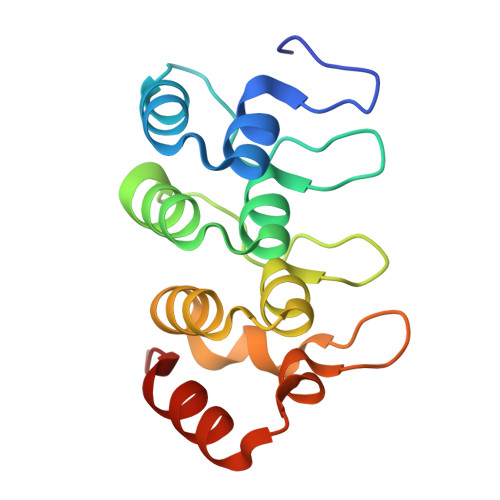
Structural mechanisms of SLF1 interactions with Histone H4 and RAD18 at the stalled replication fork.
Ryder, E.L., Nasir, N., Durgan, A.E.O., Jenkyn-Bedford, M., Tye, S., Zhang, X., Wu, Q.(2024) Nucleic Acids Res 52: 12405-12421
- PubMed: 39360622
- DOI: https://doi.org/10.1093/nar/gkae831
- Primary Citation of Related Structures:
8PEF - PubMed Abstract:
DNA damage that obstructs the replication machinery poses a significant threat to genome stability. Replication-coupled repair mechanisms safeguard stalled replication forks by coordinating proteins involved in the DNA damage response (DDR) and replication. SLF1 (SMC5-SMC6 complex localization factor 1) is crucial for facilitating the recruitment of the SMC5/6 complex to damage sites through interactions with SLF2, RAD18, and nucleosomes. However, the structural mechanisms of SLF1's interactions are unclear. In this study, we determined the crystal structure of SLF1's ankyrin repeat domain bound to an unmethylated histone H4 tail, illustrating how SLF1 reads nascent nucleosomes. Using structure-based mutagenesis, we confirmed a phosphorylation-dependent interaction necessary for a stable complex between SLF1's tandem BRCA1 C-Terminal domain (tBRCT) and the phosphorylated C-terminal region (S442 and S444) of RAD18. We validated a functional role of conserved phosphate-binding residues in SLF1, and hydrophobic residues in RAD18 that are adjacent to phosphorylation sites, both of which contribute to the strong interaction. Interestingly, we discovered a DNA-binding property of this RAD18-binding interface, providing an additional domain of SLF1 to enhance binding to nucleosomes. Our results provide critical structural insights into SLF1's interactions with post-replicative chromatin and phosphorylation-dependent DDR signalling, enhancing our understanding of SMC5/6 recruitment and/or activity during replication-coupled DNA repair.
- Astbury Centre for Structural Molecular Biology, School of Molecular & Cellular Biology, Faculty of Biological Sciences, University of Leeds, Leeds LS2 9JT, UK.
Organizational Affiliation: