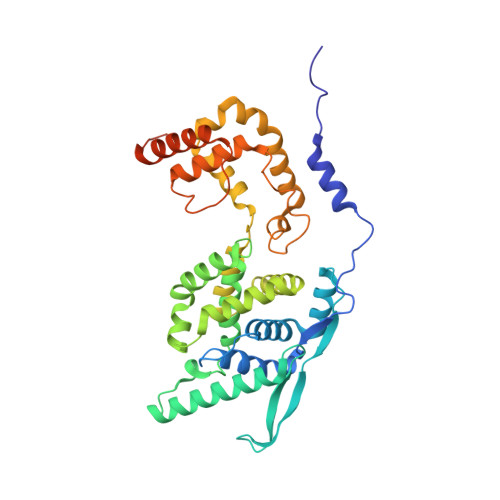
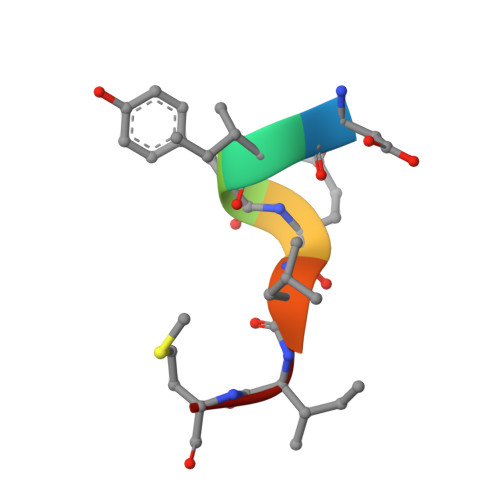
Structure of the N-RNA/P interface indicates mode of L/P recruitment to the nucleocapsid of human metapneumovirus.
Whitehead, J.D., Decool, H., Leyrat, C., Carrique, L., Fix, J., Eleouet, J.F., Galloux, M., Renner, M.(2023) Nat Commun 14: 7627-7627
- PubMed: 37993464
- DOI: https://doi.org/10.1038/s41467-023-43434-5
- Primary Citation of Related Structures:
8PDL, 8PDM, 8PDN, 8PDO, 8PDP, 8PDQ, 8PDR, 8PDS - PubMed Abstract:
Human metapneumovirus (HMPV) is a major cause of respiratory illness in young children. The HMPV polymerase (L) binds an obligate cofactor, the phosphoprotein (P). During replication and transcription, the L/P complex traverses the viral RNA genome, which is encapsidated within nucleoproteins (N). An essential interaction between N and a C-terminal region of P tethers the L/P polymerase to the template. This N-P interaction is also involved in the formation of cytoplasmic viral factories in infected cells, called inclusion bodies. To define how the polymerase component P recognizes N-encapsidated RNA (N-RNA) we employed cryogenic electron microscopy (cryo-EM) and molecular dynamics simulations, coupled to activity assays and imaging of inclusion bodies in cells. We report a 2.9 Å resolution structure of a triple-complex between multimeric N, bound to both RNA and the C-terminal region of P. Furthermore, we also present cryo-EM structures of assembled N in different oligomeric states, highlighting the plasticity of N. Combined with our functional assays, these structural data delineate in molecular detail how P attaches to N-RNA whilst retaining substantial conformational dynamics. Moreover, the N-RNA-P triple complex structure provides a molecular blueprint for the design of therapeutics to potentially disrupt the attachment of L/P to its template.
- Division of Structural Biology, The Wellcome Centre for Human Genetics, University of Oxford, Oxford, UK.
Organizational Affiliation: