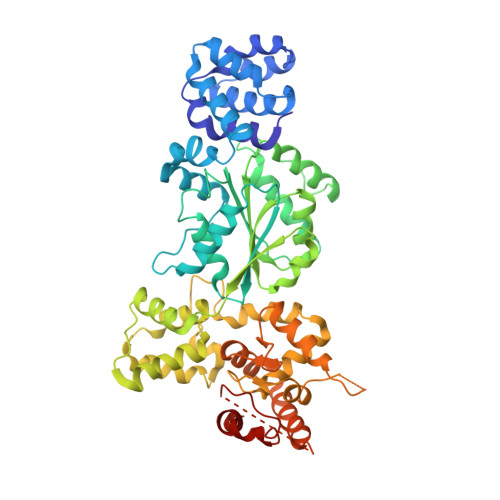
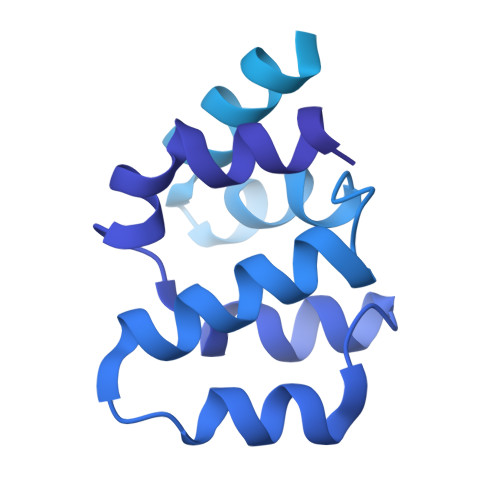
Structural insights into CED-3 activation.
Li, Y., Tian, L., Zhang, Y., Shi, Y.(2023) Life Sci Alliance 6
- PubMed: 37402593
- DOI: https://doi.org/10.26508/lsa.202302056
- Primary Citation of Related Structures:
8JNS, 8JO0, 8JOL - PubMed Abstract:
In Caenorhabditis elegans (C. elegans) , onset of programmed cell death is marked with the activation of CED-3, a process that requires assembly of the CED-4 apoptosome. Activated CED-3 forms a holoenzyme with the CED-4 apoptosome to cleave a wide range of substrates, leading to irreversible cell death. Despite decades of investigations, the underlying mechanism of CED-4-facilitated CED-3 activation remains elusive. Here, we report cryo-EM structures of the CED-4 apoptosome and three distinct CED-4/CED-3 complexes that mimic different activation stages for CED-3. In addition to the previously reported octamer in crystal structures, CED-4, alone or in complex with CED-3, exists in multiple oligomeric states. Supported by biochemical analyses, we show that the conserved CARD-CARD interaction promotes CED-3 activation, and initiation of programmed cell death is regulated by the dynamic organization of the CED-4 apoptosome.
- Beijing Frontier Research Center for Biological Structures, Tsinghua-Peking Center for Life Sciences, School of Life Sciences, Tsinghua University, Beijing, China liyini@mail.tsinghua.edu.cn.
Organizational Affiliation: