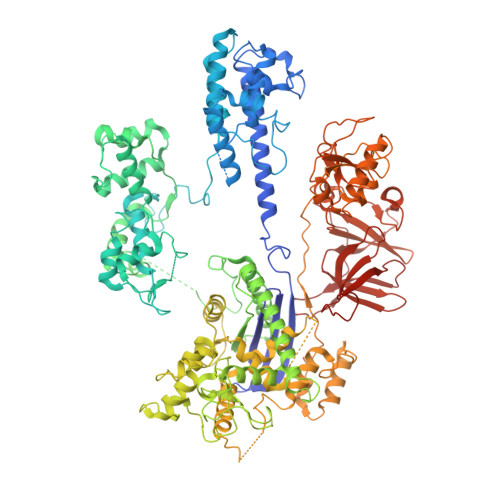
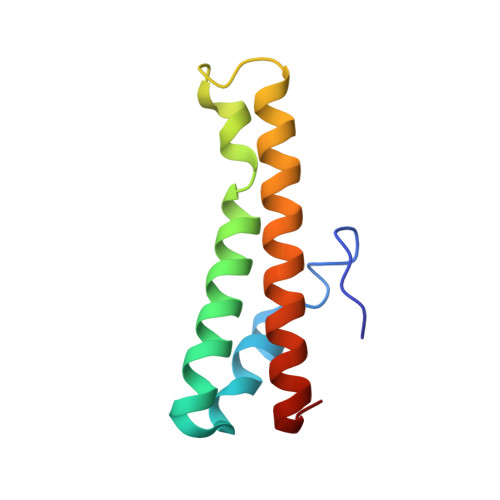
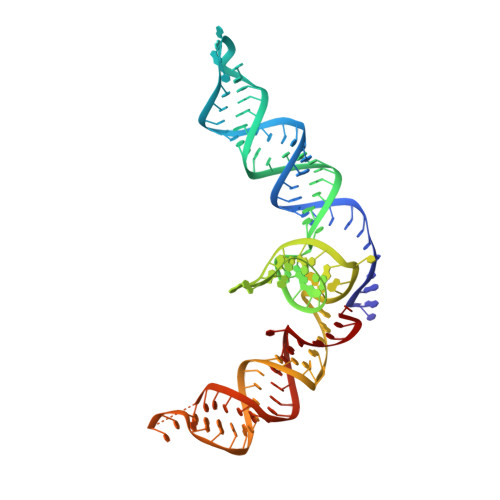
Inhibitory mechanism of CRISPR-Cas9 by AcrIIC4.
Li, X., Liao, F., Gao, J., Song, G., Zhang, C., Ji, N., Wang, X., Wen, J., He, J., Wei, Y., Zhang, H., Li, Z., Yu, G., Yin, H.(2023) Nucleic Acids Res 51: 9442-9451
- PubMed: 37587688
- DOI: https://doi.org/10.1093/nar/gkad669
- Primary Citation of Related Structures:
7XVQ, 8JA0 - PubMed Abstract:
CRISPR-Cas systems act as the adaptive immune systems of bacteria and archaea, targeting and destroying invading foreign mobile genetic elements (MGEs) such as phages. MGEs have also evolved anti-CRISPR (Acr) proteins to inactivate the CRISPR-Cas systems. Recently, AcrIIC4, identified from Haemophilus parainfluenzae phage, has been reported to inhibit the endonuclease activity of Cas9 from Neisseria meningitidis (NmeCas9), but the inhibition mechanism is not clear. Here, we biochemically and structurally investigated the anti-CRISPR activity of AcrIIC4. AcrIIC4 folds into a helix bundle composed of three helices, which associates with the REC lobe of NmeCas9 and sgRNA. The REC2 domain of NmeCas9 is locked by AcrIIC4, perturbing the conformational dynamics required for the target DNA binding and cleavage. Furthermore, mutation of the key residues in the AcrIIC4-NmeCas9 and AcrIIC4-sgRNA interfaces largely abolishes the inhibitory effects of AcrIIC4. Our study offers new insights into the mechanism of AcrIIC4-mediated suppression of NmeCas9 and provides guidelines for the design of regulatory tools for Cas9-based gene editing applications.
- State Key Laboratory of Experimental Hematology, Key Laboratory of Immune Microenvironment and Disease (Ministry of Education), The Province and Ministry Co-sponsored Collaborative Innovation Center for Medical Epigenetics, Haihe Laboratory of Cell Ecosystem, Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Tianjin Medical University, Tianjin 300070, China.
Organizational Affiliation: