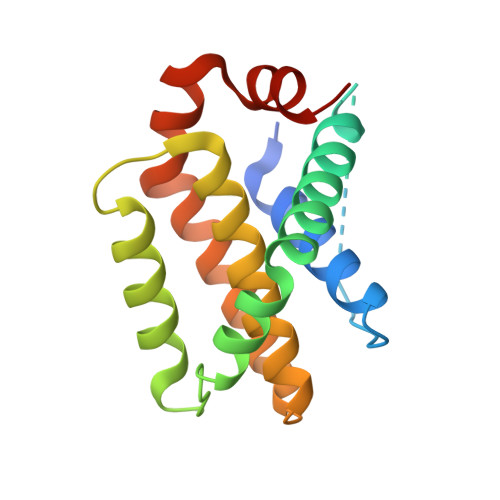
Structural basis of the specificity and interaction mechanism of Bmf binding to pro-survival Bcl-2 family proteins.
Wang, H., Guo, M., Wei, H., Chen, Y.(2023) Comput Struct Biotechnol J 21: 3760-3767
- PubMed: 37560128
- DOI: https://doi.org/10.1016/j.csbj.2023.07.017
- Primary Citation of Related Structures:
8IQK, 8IQL, 8IQM - PubMed Abstract:
The apoptotic pathway is regulated by protein-protein interactions between members of the Bcl-2 family. Pro-survival Bcl-2 family proteins act as cell guardians and protect cells against death. Selective binding and neutralization of BH3-only proteins with pro-survival Bcl-2 family proteins is critical for initiating apoptosis. In this study, the binding assay shows that the BH3 peptide derived from the BH3-only protein Bmf has a high affinity for the pro-survival proteins Bcl-2 and Bcl-xL, but a much lower affinity for Mcl-1. The complex structures of Bmf BH3 with Bcl-2, Bcl-xL and Mcl-1 reveal that the α-helical Bmf BH3 accommodates into the canonical groove of these pro-survival proteins, but the conformational changes and some interactions are different among the three complexes. Bmf BH3 forms conserved hydrophobic and salt bridge interactions with Bcl-2 and Bcl-xL, and also establishes several hydrogen bonds to support their binding. However, the highly conserved Asp-Arg salt bridge is not formed in the Mcl-1/Bmf BH3 complex, and few hydrogen bonds are observed. Furthermore, mutational analysis shows that substitutions of less-conserved residues in the α2-α3 region of these pro-survival Bcl-2 family proteins, as well as the highly conserved Arg, lead to significant changes in their binding affinity to Bmf BH3, while substitutions of less-conserved residues in Bmf BH3 have a more dramatic effect on its affinity to Mcl-1. This study provides structural insight into the specificity and interaction mechanism of Bmf BH3 binding to pro-survival Bcl-2 family proteins, and helps guide the design of BH3 mimics targeting pro-survival Bcl-2 family proteins.
- Department of Oncology, NHC Key Laboratory of Cancer Proteomics, Laboratory of Structural Biology, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan 410008, China.
Organizational Affiliation: