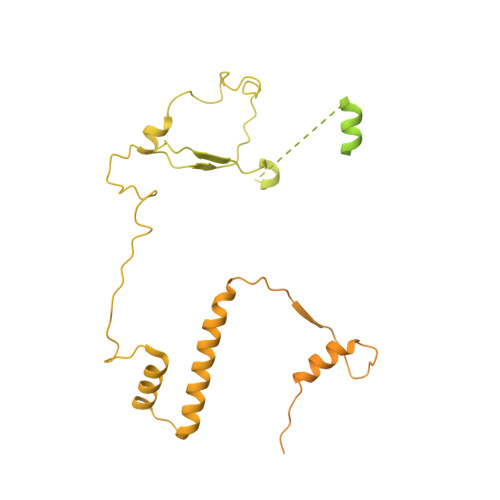
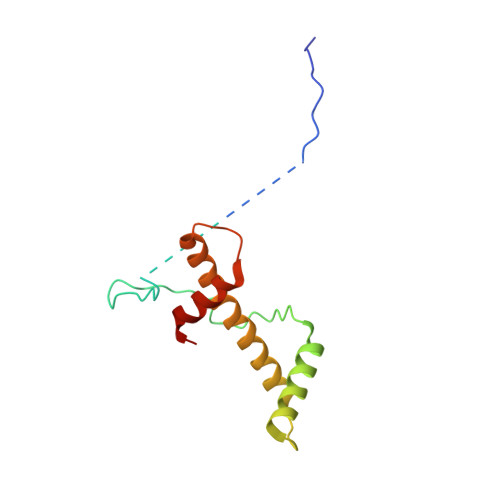
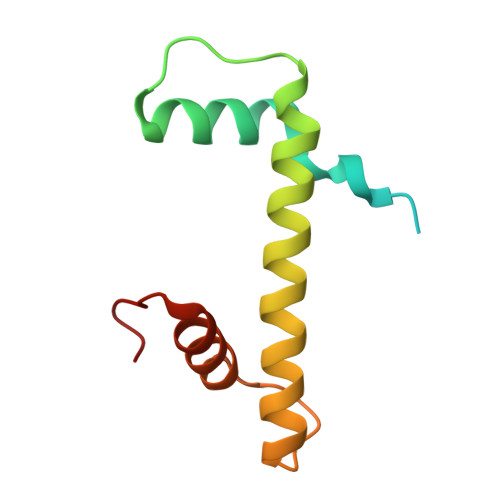
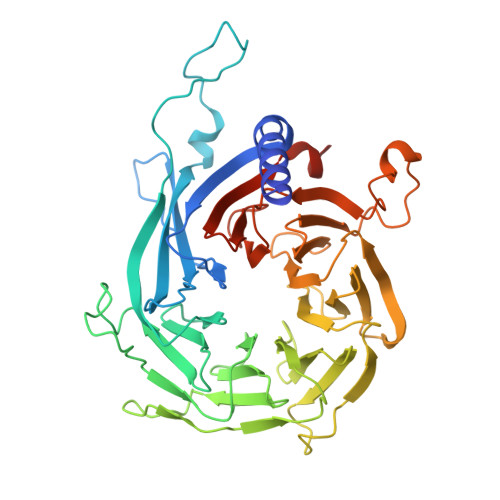
Structural insights into histone binding and nucleosome assembly by chromatin assembly factor-1.
Liu, C.P., Yu, Z., Xiong, J., Hu, J., Song, A., Ding, D., Yu, C., Yang, N., Wang, M., Yu, J., Hou, P., Zeng, K., Li, Z., Zhang, Z., Zhang, X., Li, W., Zhang, Z., Zhu, B., Li, G., Xu, R.M.(2023) Science 381: eadd8673-eadd8673
- PubMed: 37616371
- DOI: https://doi.org/10.1126/science.add8673
- Primary Citation of Related Structures:
7Y5K, 7Y5L, 7Y5O, 7Y5U, 7Y5V, 7Y5W, 7Y60, 7Y61, 8IQF, 8IQG, 8J6S, 8J6T - PubMed Abstract:
Chromatin inheritance entails de novo nucleosome assembly after DNA replication by chromatin assembly factor-1 (CAF-1). Yet direct knowledge about CAF-1's histone binding mode and nucleosome assembly process is lacking. In this work, we report the crystal structure of human CAF-1 in the absence of histones and the cryo-electron microscopy structure of CAF-1 in complex with histones H3 and H4. One histone H3-H4 heterodimer is bound by one CAF-1 complex mainly through the p60 subunit and the acidic domain of the p150 subunit. We also observed a dimeric CAF-1-H3-H4 supercomplex in which two H3-H4 heterodimers are poised for tetramer assembly and discovered that CAF-1 facilitates right-handed DNA wrapping of H3-H4 tetramers. These findings signify the involvement of DNA in H3-H4 tetramer formation and suggest a right-handed nucleosome precursor in chromatin replication.
- National Laboratory of Biomacromolecules and Key Laboratory of Epigenetic Regulation and Intervention, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China.
Organizational Affiliation: