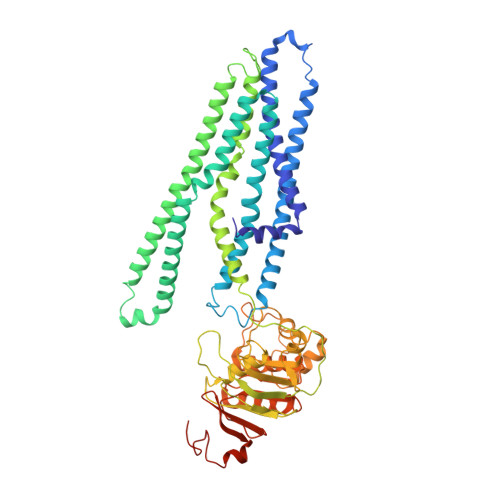
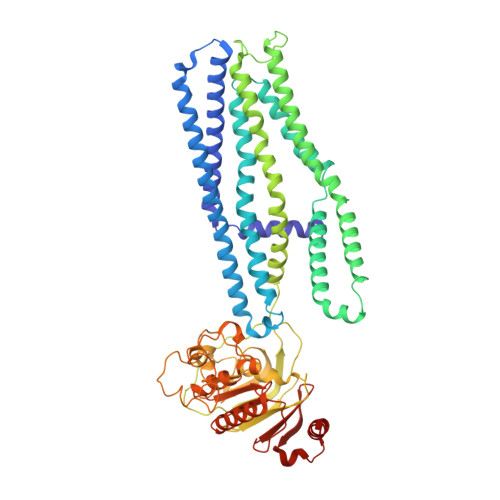
Cryo-EM structures of a prokaryotic heme transporter CydDC.
Zhu, C., Shi, Y., Yu, J., Zhao, W., Li, L., Liang, J., Yang, X., Zhang, B., Zhao, Y., Gao, Y., Chen, X., Yang, X., Zhang, L., Guddat, L.W., Liu, L., Yang, H., Rao, Z., Li, J.(2023) Protein Cell 14: 919-923