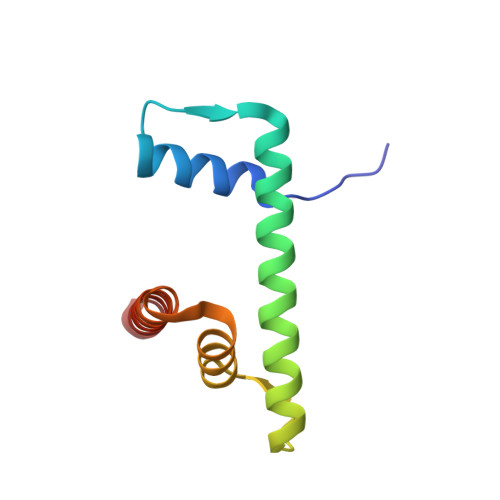
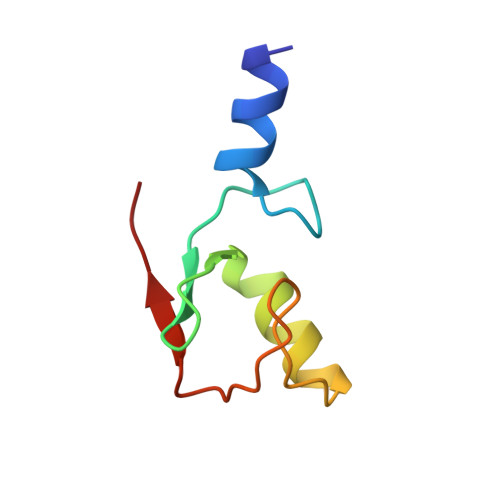
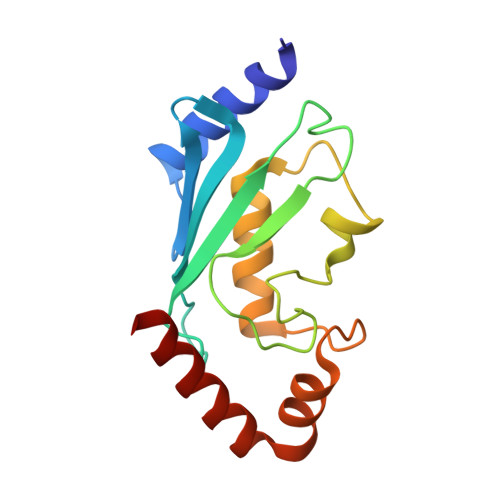
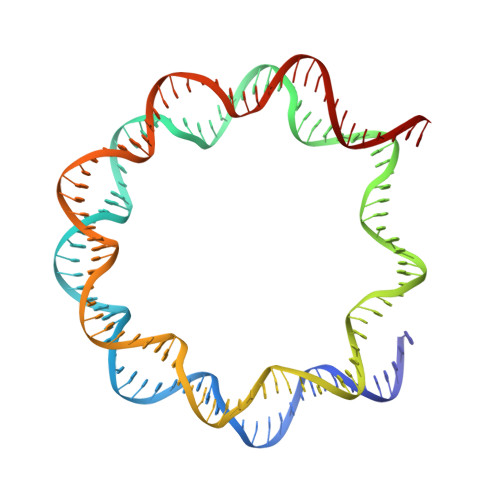
Mechanistic insights into nucleosomal H2B monoubiquitylation mediated by yeast Bre1-Rad6 and its human homolog RNF20/RNF40-hRAD6A.
Deng, Z., Ai, H., Sun, M., Tong, Z., Du, Y., Qu, Q., Zhang, L., Xu, Z., Tao, S., Shi, Q., Li, J.B., Pan, M., Liu, L.(2023) Mol Cell 83: 3080-3094.e14
- PubMed: 37633270
- DOI: https://doi.org/10.1016/j.molcel.2023.08.001
- Primary Citation of Related Structures:
8IEG, 8IEJ - PubMed Abstract:
Histone H2B monoubiquitylation plays essential roles in chromatin-based transcriptional processes. A RING-type E3 ligase (yeast Bre1 or human RNF20/RNF40) and an E2 ubiquitin-conjugating enzyme (yeast Rad6 or human hRAD6A), together, precisely deposit ubiquitin on H2B K123 in yeast or K120 in humans. Here, we developed a chemical trapping strategy and successfully captured the transient structures of Bre1- or RNF20/RNF40-mediated ubiquitin transfer from Rad6 or hRAD6A to nucleosomal H2B. Our structures show that Bre1 and RNF40 directly bind nucleosomal DNA, exhibiting a conserved E3/E2/nucleosome interaction pattern from yeast to humans for H2B monoubiquitylation. We also find an uncanonical non-hydrophobic contact in the Bre1 RING-Rad6 interface, which positions Rad6 directly above the target H2B lysine residue. Our study provides mechanistic insights into the site-specific monoubiquitylation of H2B, reveals a critical role of nucleosomal DNA in mediating E3 ligase recognition, and provides a framework for understanding the cancer-driving mutations of RNF20/RNF40.
- Tsinghua-Peking Joint Center for Life Sciences, MOE Key Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology, Center for Synthetic and Systems Biology, Department of Chemistry, Tsinghua University, Beijing 100084, China.
Organizational Affiliation: