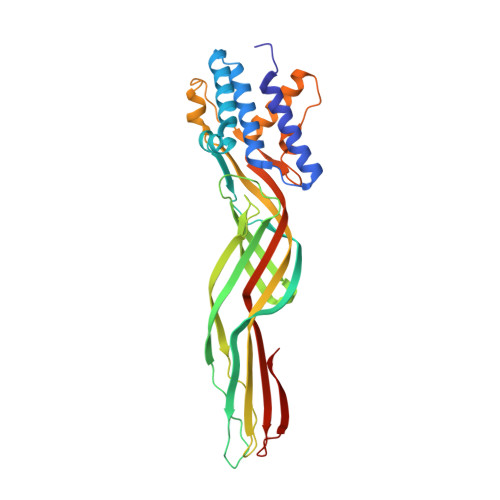
Structural insight into Bacillus thuringiensis Sip1Ab reveals its similarity to ETX_MTX2 family beta-pore-forming toxin.
Chen, Z., Shi, Y., Wang, D., Liu, X., Jiao, X., Gao, X., Jiang, K.(2023) Pest Manag Sci 79: 4264-4273
- PubMed: 37341620
- DOI: https://doi.org/10.1002/ps.7622
- Primary Citation of Related Structures:
8HX3 - PubMed Abstract:
Microbially derived, protein-based biopesticides are an important approach for sustainable pest management. The secreted insecticidal proteins (Sips) produced by the bacterium Bacillus thuringiensis exhibit potent insecticidal activity against coleopteran pests and are, therefore, attractive as candidate biopesticides. However, the modes-of-action of Sips are unclear as comprehensive structural information for these proteins is lacking. Using X-ray crystallography, we elucidated the structure of monomeric Sip1Ab at 2.28 Å resolution. Structural analyses revealed that Sip1Ab has the three domains and conserved fold characteristic of other aerolysin-like beta-pore-forming toxins (β-PFTs). Based on the sequence and structural similarities between Sip1Ab and other ETX_MTX2 subfamily toxins, we suggested the mechanism of these proteins and proposed that it is common to them all. The atomic-level structural data for Sip1Ab generated by the present study could facilitate future structural and mechanistic research on Sips as well as their application in sustainable insect pest management. © 2023 Society of Chemical Industry.
- State Key Laboratory of Microbial Technology, Shandong University, Qingdao, China.
Organizational Affiliation: