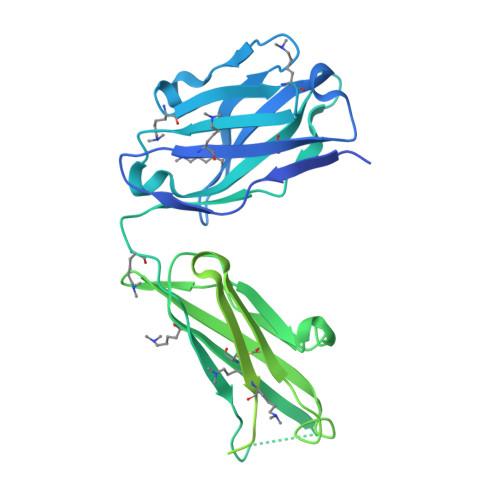
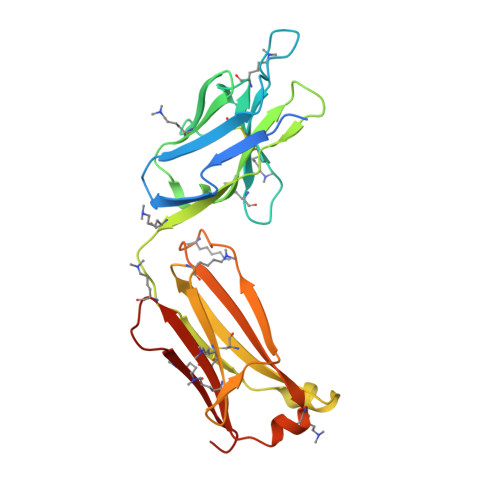
Structural and molecular insight into antibody recognition of dynamic neoepitopes in membrane tethered MUC1 of pancreatic cancer cells and secreted exosomes.
Wakui, H., Yokoi, Y., Horidome, C., Ose, T., Yao, M., Tanaka, Y., Hinou, H., Nishimura, S.I.(2023) RSC Chem Biol 4: 564-572
- PubMed: 37547453
- DOI: https://doi.org/10.1039/d3cb00036b
- Primary Citation of Related Structures:
8HRH - PubMed Abstract:
Pancreatic cancer is highly metastatic and has poor prognosis, mainly due to delayed detection, often after metastasis has occurred. A novel method to enable early detection and disease intervention is strongly needed. Here we unveil for the first time that pancreatic cancer cells (PANC-1) and secreted exosomes express MUC1 bearing cancer-relevant dynamic epitopes recognized specifically by an anti-MUC1 antibody (SN-131), which binds specifically core 1 but not core 2 type O -glycans found in normal cells. Comprehensive assessment of the essential epitope for SN-131 indicates that PANC-1 cells produce dominantly MUC1 with aberrant O -glycoforms such as Tn, T, and sialyl T (ST) antigens. Importantly, SN-131 showed the highest affinity with MUC1 bearing ST antigen at the immunodominant DTR motif ( K D = 1.58 nM) independent of the glycosylation states of other Ser/Thr residues in the MUC1 tandem repeats. The X-ray structure revealed that SN-131 interacts directly with Neu5Ac and root GalNAc of the ST antigen in addition to the proximal peptide region. Our results demonstrate that targeting O -glycosylated "dynamic neoepitopes" found in the membrane-tethered MUC1 is a promising therapeutic strategy for improving the treatment outcome of patients with pancreatic cancer.
- Field of Drug Discovery Research, Faculty of Advanced Life Science, and Graduate School of Life Science, Hokkaido University N21 W11 Kita-ku Sapporo 001-0021 Japan shin@sci.hokudai.ac.jp.
Organizational Affiliation: