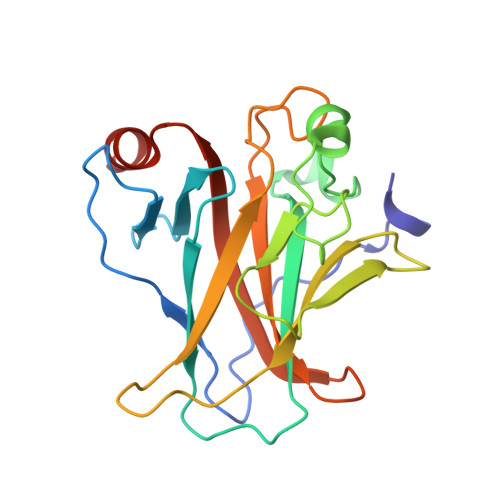
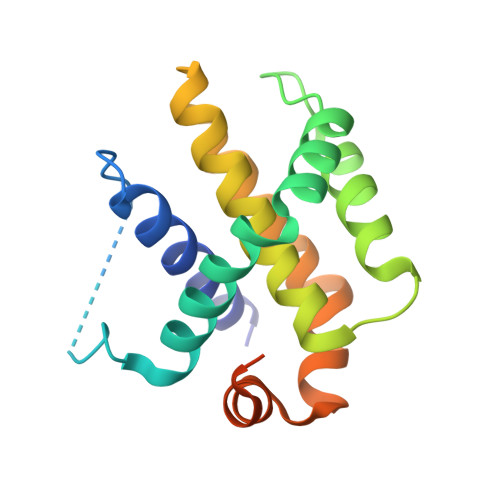
Structures of p53/BCL-2 complex suggest a mechanism for p53 to antagonize BCL-2 activity.
Wei, H., Wang, H., Wang, G., Qu, L., Jiang, L., Dai, S., Chen, X., Zhang, Y., Chen, Z., Li, Y., Guo, M., Chen, Y.(2023) Nat Commun 14: 4300-4300
- PubMed: 37463921
- DOI: https://doi.org/10.1038/s41467-023-40087-2
- Primary Citation of Related Structures:
8HLL, 8HLM, 8HLN - PubMed Abstract:
Mitochondrial apoptosis is strictly controlled by BCL-2 family proteins through a subtle network of protein interactions. The tumor suppressor protein p53 triggers transcription-independent apoptosis through direct interactions with BCL-2 family proteins, but the molecular mechanism is not well understood. In this study, we present three crystal structures of p53-DBD in complex with the anti-apoptotic protein BCL-2 at resolutions of 2.3-2.7 Å. The structures show that two loops of p53-DBD penetrate directly into the BH3-binding pocket of BCL-2. Structure-based mutations at the interface impair the p53/BCL-2 interaction. Specifically, the binding sites for p53 and the pro-apoptotic protein Bax in the BCL-2 pocket are mostly identical. In addition, formation of the p53/BCL-2 complex is negatively correlated with the formation of BCL-2 complexes with pro-apoptotic BCL-2 family members. Defects in the p53/BCL-2 interaction attenuate p53-mediated cell apoptosis. Overall, our study provides a structural basis for the interaction between p53 and BCL-2, and suggests a molecular mechanism by which p53 regulates transcription-independent apoptosis by antagonizing the interaction of BCL-2 with pro-apoptotic BCL-2 family members.
- Department of Oncology, NHC Key Laboratory of Cancer Proteomics & State Local Joint Engineering Laboratroy for Anticancer Drugs, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, 410008, China.
Organizational Affiliation: