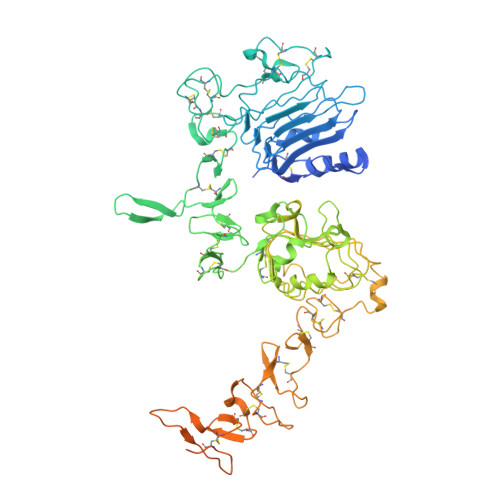
Structure and dynamics of the EGFR/HER2 heterodimer.
Bai, X., Sun, P., Wang, X., Long, C., Liao, S., Dang, S., Zhuang, S., Du, Y., Zhang, X., Li, N., He, K., Zhang, Z.(2023) Cell Discov 9: 18-18
- PubMed: 36781849
- DOI: https://doi.org/10.1038/s41421-023-00523-5
- Primary Citation of Related Structures:
8HGO, 8HGP, 8HGS - PubMed Abstract:
HER2 belongs to the human epidermal growth factor receptor tyrosine kinase family. Its overexpression or hyperactivation is a leading cause for multiple types of cancers. HER2 functions mainly through dimerization with other family members, such as EGFR. However, the molecular details for heterodimer assembly have not been completely understood. Here, we report cryo-EM structures of the EGF- and epiregulin-bound EGFR/HER2 ectodomain complexes at resolutions of 3.3 Å and 4.5 Å, respectively. Together with the functional analyses, we demonstrate that only the dimerization arm of HER2, but not that of EGFR, is essential for their heterodimer formation and signal transduction. Moreover, we analyze the differential membrane dynamics and transient interactions of endogenous EGFR and HER2 molecules in genome-edited cells using single-molecule live-cell imaging. Furthermore, we show that the interaction with HER2 could allow EGFR to resist endocytosis. Together, this work deepens our understanding of the unique structural properties and dynamics of the EGFR/HER2 complex.
- State Key Laboratory of Membrane Biology, School of Life Sciences, Peking University, Beijing, China.
Organizational Affiliation: