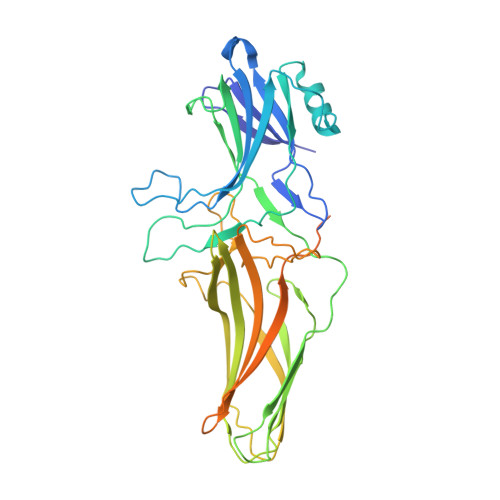
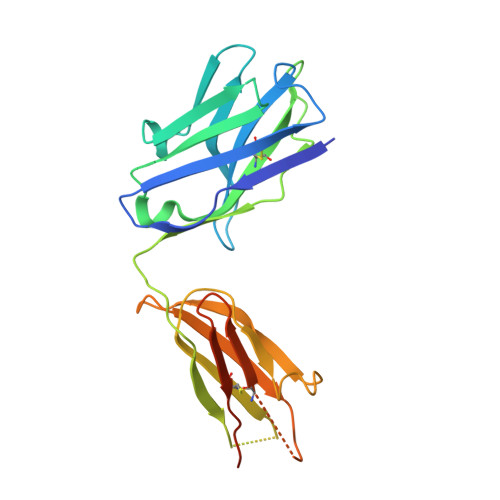
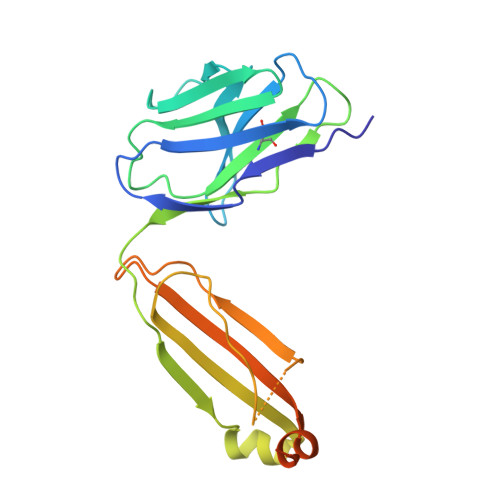
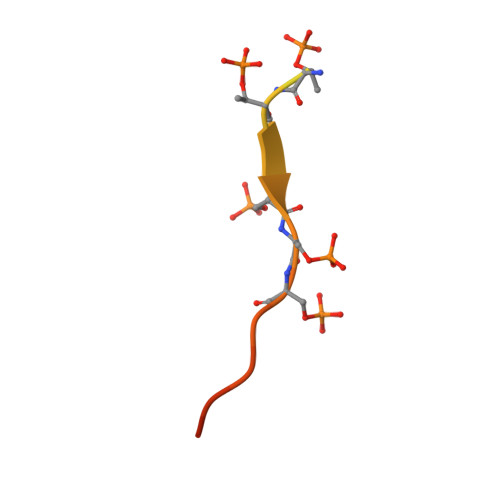
Structural snapshots uncover a key phosphorylation motif in GPCRs driving beta-arrestin activation.
Maharana, J., Sarma, P., Yadav, M.K., Saha, S., Singh, V., Saha, S., Chami, M., Banerjee, R., Shukla, A.K.(2023) Mol Cell 83: 2091-2107.e7
- PubMed: 37209686
- DOI: https://doi.org/10.1016/j.molcel.2023.04.025
- Primary Citation of Related Structures:
8GO8, 8GOC, 8GOO, 8GP3, 8I0N, 8I0Q, 8I0Z, 8I10 - PubMed Abstract:
Agonist-induced GPCR phosphorylation is a key determinant for the binding and activation of β-arrestins (βarrs). However, it is not entirely clear how different GPCRs harboring divergent phosphorylation patterns impart converging active conformation on βarrs leading to broadly conserved functional responses such as desensitization, endocytosis, and signaling. Here, we present multiple cryo-EM structures of activated βarrs in complex with distinct phosphorylation patterns derived from the carboxyl terminus of different GPCRs. These structures help identify a P-X-P-P type phosphorylation motif in GPCRs that interacts with a spatially organized K-K-R-R-K-K sequence in the N-domain of βarrs. Sequence analysis of the human GPCRome reveals the presence of this phosphorylation pattern in a large number of receptors, and its contribution in βarr activation is demonstrated by targeted mutagenesis experiments combined with an intrabody-based conformational sensor. Taken together, our findings provide important structural insights into the ability of distinct GPCRs to activate βarrs through a significantly conserved mechanism.
- Department of Biological Sciences and Bioengineering, Indian Institute of Technology, Kanpur 208016, India.
Organizational Affiliation: