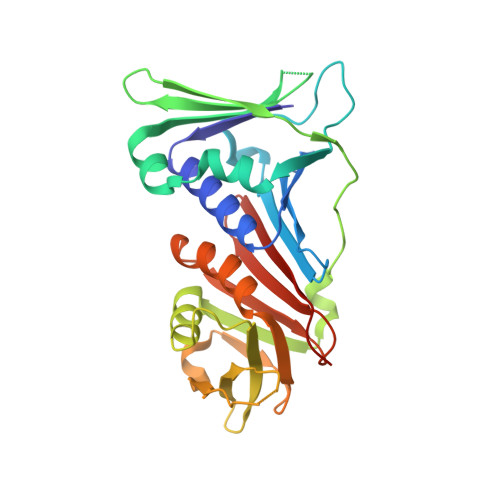
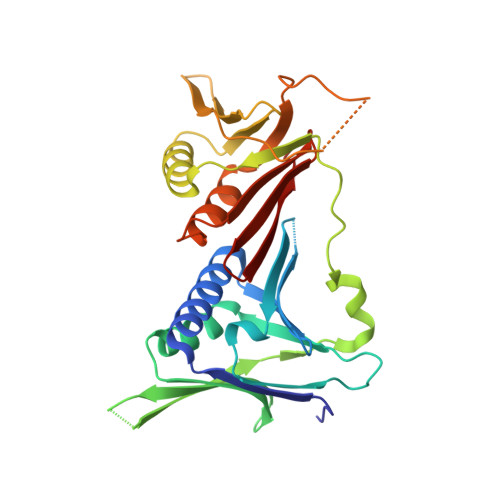
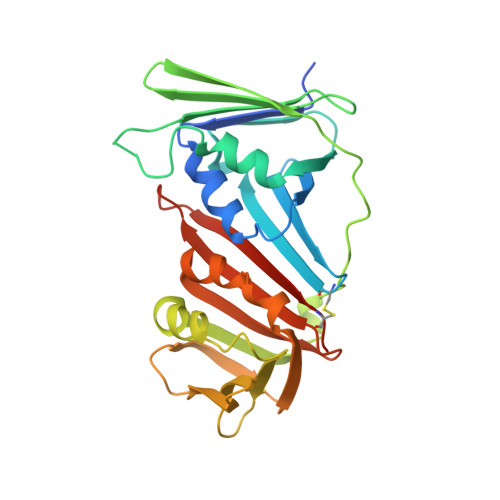
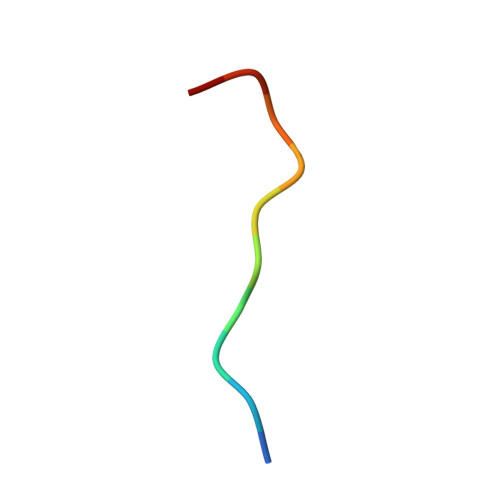
The 9-1-1 DNA clamp subunit RAD1 forms specific interactions with clamp loader RAD17, revealing functional implications for binding-protein RHINO.
Hara, K., Hishiki, A., Hoshino, T., Nagata, K., Iida, N., Sawada, Y., Ohashi, E., Hashimoto, H.(2023) J Biological Chem 299: 103061-103061
- PubMed: 36841485
- DOI: https://doi.org/10.1016/j.jbc.2023.103061
- Primary Citation of Related Structures:
8GNN - PubMed Abstract:
The RAD9-RAD1-HUS1 complex (9-1-1) is a eukaryotic DNA clamp with a crucial role at checkpoints for DNA damage. The ring-like structure of 9-1-1 is opened for loading onto 5' recessed DNA by the clamp loader RAD17 RFC-like complex (RAD17-RLC), in which the RAD17 subunit is responsible for specificity to 9-1-1. Loading of 9-1-1 is required for activation of the ATR-CHK1 checkpoint pathway and the activation is stimulated by a 9-1-1 interacting protein, RHINO, which interacts with 9-1-1 via a recently identified RAD1-binding motif. This discovery led to the hypothesis that other interacting proteins may contain a RAD1-binding motif as well. Here, we show that vertebrate RAD17 proteins also have a putative RAD1-binding motif in their N-terminal regions, and we report the crystal structure of human 9-1-1 bound to a human RAD17 peptide incorporating the motif at 2.1 Å resolution. Our structure confirms that the N-terminal region of RAD17 binds to the RAD1 subunit of 9-1-1 via specific interactions. Furthermore, we show that the RAD1-binding motif of RHINO disturbs the interaction of the N-terminal region of RAD17 with 9-1-1. Our results provide deeper understanding of how RAD17-RLC specifically recognizes 9-1-1 and imply that RHINO has a functional role in 9-1-1 loading/unloading and checkpoint activation.
- School of Pharmaceutical Sciences, University of Shizuoka, Shizuoka, Japan.
Organizational Affiliation: