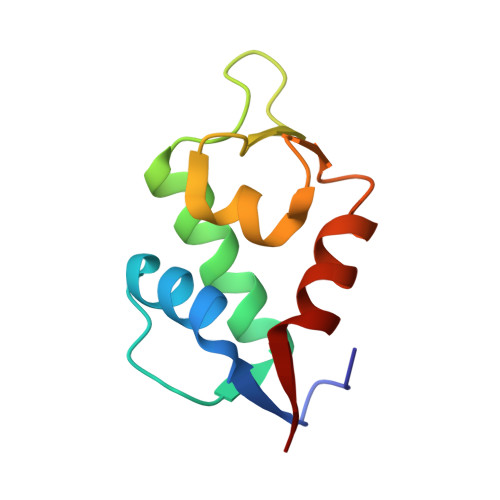
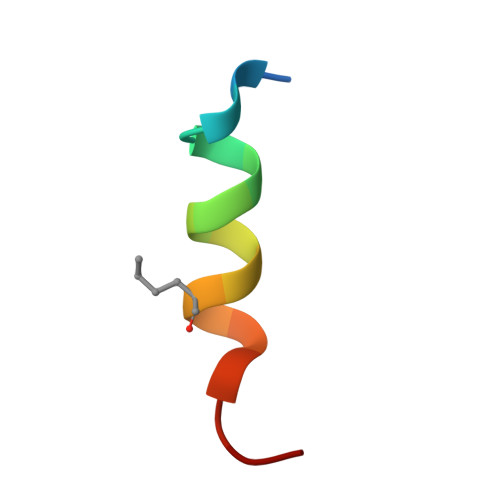
Discovery of Sulanemadlin (ALRN-6924), the First Cell-Permeating, Stabilized alpha-Helical Peptide in Clinical Development.
Guerlavais, V., Sawyer, T.K., Carvajal, L., Chang, Y.S., Graves, B., Ren, J.G., Sutton, D., Olson, K.A., Packman, K., Darlak, K., Elkin, C., Feyfant, E., Kesavan, K., Gangurde, P., Vassilev, L.T., Nash, H.M., Vukovic, V., Aivado, M., Annis, D.A.(2023) J Med Chem 66: 9401-9417
- PubMed: 37439511
- DOI: https://doi.org/10.1021/acs.jmedchem.3c00623
- Primary Citation of Related Structures:
8GJS - PubMed Abstract:
We report the discovery of sulanemadlin (ALRN-6924), the first cell-permeating, stabilized α-helical peptide to enter clinical trials. ALRN-6924 is a "stapled peptide" that mimics the N-terminal domain of the p53 tumor suppressor protein. It binds with high affinity to both MDM2 and MDMX (also known as MDM4), the endogenous inhibitors of p53, to activate p53 signaling in cells having a non-mutant, or wild-type TP53 genotype ( TP53 -WT). Iterative structure-activity optimization endowed ALRN-6924 with favorable cell permeability, solubility, and pharmacokinetic and safety profiles. Intracellular proteolysis of ALRN-6924 forms a long-acting active metabolite with potent MDM2 and MDMX binding affinity and slow dissociation kinetics. At high doses, ALRN-6924 exhibits on-mechanism anticancer activity in TP53 -WT tumor models. At lower doses, ALRN-6924 transiently arrests the cell cycle in healthy tissues to protect them from chemotherapy without protecting the TP53 -mutant cancer cells. These results support the continued clinical evaluation of ALRN-6924 as an anticancer and chemoprotection agent.
- Aileron Therapeutics, Inc., 738 Main Street #398, Waltham, Massachusetts 02451, United States.
Organizational Affiliation: