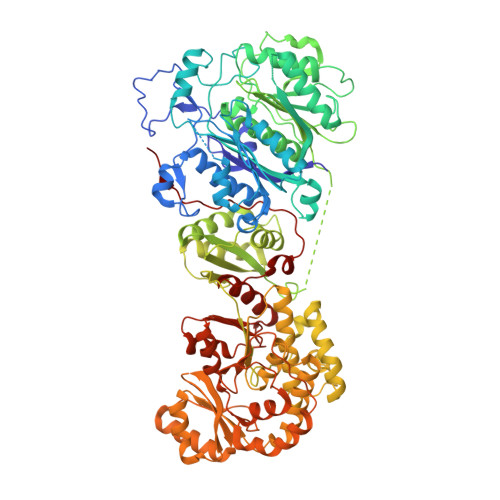
Crystal structures reveal the framework of cis -acyltransferase modular polyketide synthases.
Keatinge-Clay, A.T., Miyazawa, T., Zhang, J., Ray, K.A., Lutgens, J.D., Bista, R., Lin, S.N.(2023) bioRxiv
- PubMed: 36798387
- DOI: https://doi.org/10.1101/2023.02.11.528132
- Primary Citation of Related Structures:
8G4U - PubMed Abstract:
Although the domains of cis -acyltransferase ( cis -AT) modular polyketide synthases (PKS's) have been understood at atomic resolution for over a decade, the domain-domain interactions responsible for the architectures and activities of these giant molecular assembly lines remain largely uncharacterized. The multimeric structure of the α 6 β 6 fungal fatty acid synthase (FAS) provides 6 equivalent reaction chambers for its acyl carrier protein (ACP) domains to shuttle carbon building blocks and the growing acyl chain between surrounding, oriented enzymatic domains. The presumed homodimeric oligomerization of cis -AT assembly lines is insufficient to provide similar reaction chambers; however, the crystal structure of a ketosynthase (KS)+AT didomain presented here and three already reported show an interaction between the AT domains appropriate for lateral multimerization. This interaction was used to construct a framework for the pikromycin PKS from its KS, AT, and docking domains that contains highly-ordered reaction chambers. Its AT domains also mediate vertical interactions, both with upstream KS domains and downstream docking domains.