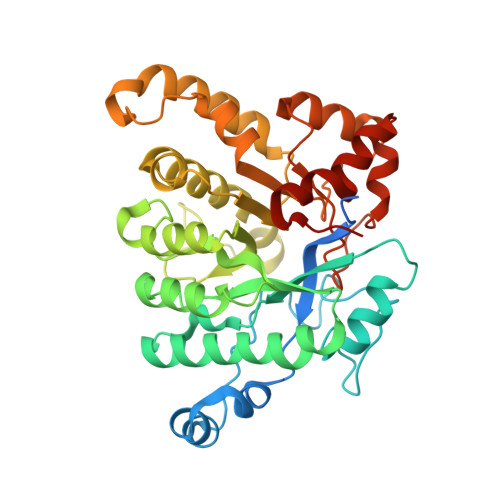
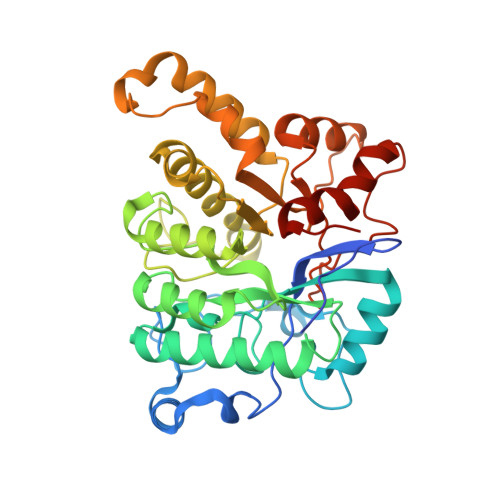
Enzymatic Hydroxylation of Aliphatic C-H Bonds by a Mn/Fe Cofactor.
Powell, M.M., Rao, G., Britt, R.D., Rittle, J.(2023) J Am Chem Soc 145: 16526-16537
- PubMed: 37471626
- DOI: https://doi.org/10.1021/jacs.3c03419
- Primary Citation of Related Structures:
8FUL, 8FUM, 8FUN, 8FUO - PubMed Abstract:
The aerobic oxidation of carbon-hydrogen (C-H) bonds in biology is currently known to be accomplished by a limited set of cofactors that typically include heme, nonheme iron, and copper. While manganese cofactors perform difficult oxidation reactions, including water oxidation within Photosystem II, they are generally not known to be used for C-H bond activation, and those that do catalyze this important reaction display limited intrinsic reactivity. Here we report that the 2-aminoisobutyric acid hydroxylase from Rhodococcus wratislaviensis , AibH1H2, requires manganese to functionalize a strong, aliphatic C-H bond (BDE = 100 kcal/mol). Structural and spectroscopic studies of this enzyme reveal a redox-active, heterobimetallic manganese-iron active site at the locus of O 2 activation and substrate coordination. This result expands the known reactivity of biological manganese-iron cofactors, which was previously restricted to single-electron transfer or stoichiometric protein oxidation. Furthermore, the AibH1H2 cofactor is supported by a protein fold distinct from typical bimetallic oxygenases, and bioinformatic analyses identify related proteins abundant in microorganisms. This suggests that many uncharacterized monooxygenases may similarly require manganese to perform oxidative biochemical tasks.
- Department of Chemistry, University of California, Berkeley, Berkeley, California 94720, United States.
Organizational Affiliation: