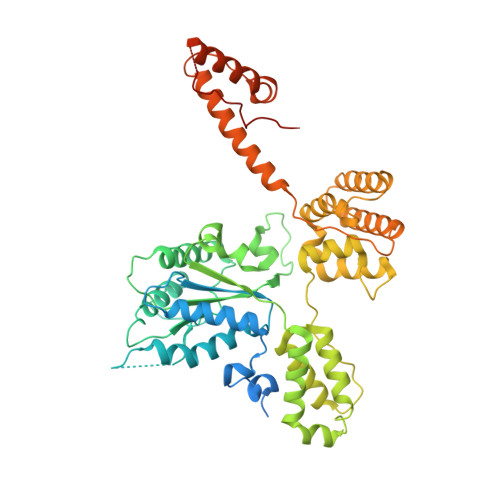
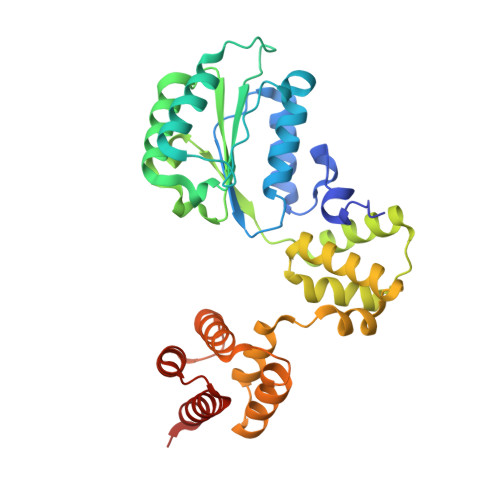
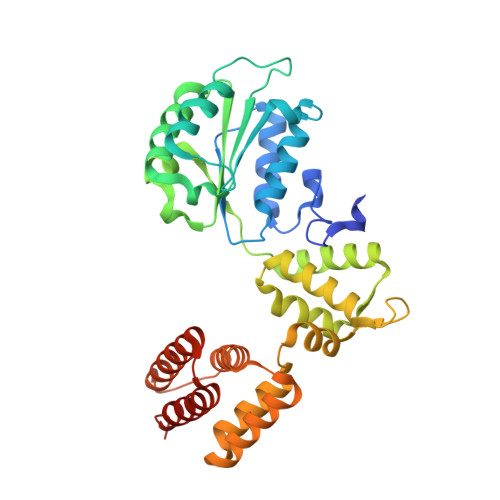
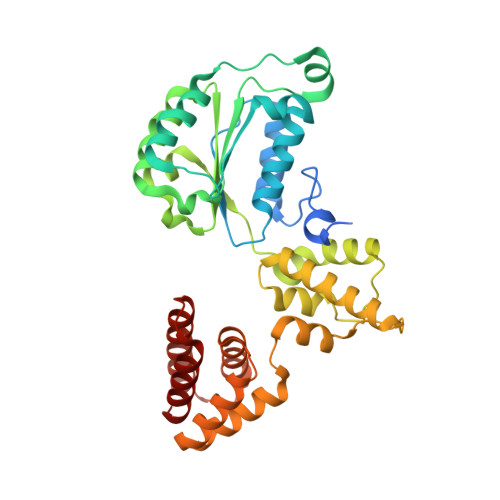
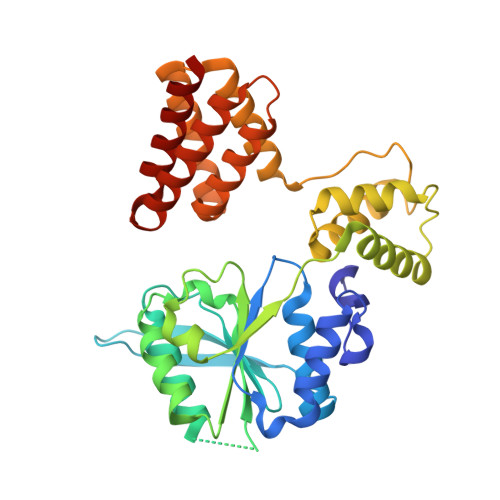
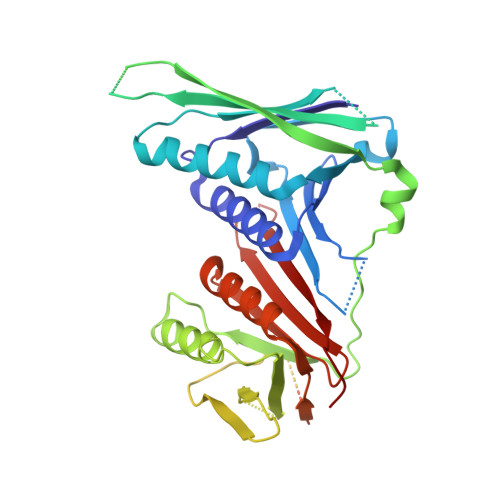
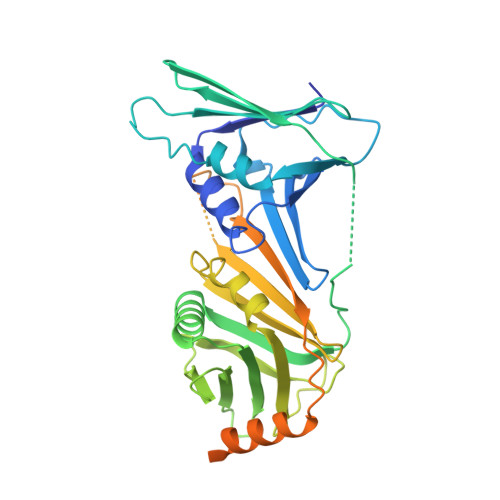
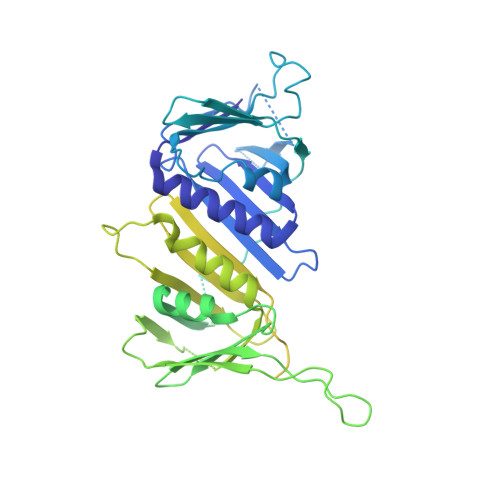
Structures of 9-1-1 DNA checkpoint clamp loading at gaps from start to finish and ramification to biology.
Zheng, F., Georgescu, R.E., Yao, N.Y., O'Donnell, M.E., Li, H.(2023) bioRxiv
- PubMed: 37205533
- DOI: https://doi.org/10.1101/2023.05.03.539266
- Primary Citation of Related Structures:
8FS3, 8FS4, 8FS5, 8FS6, 8FS7, 8FS8 - PubMed Abstract:
Recent structural studies show the Rad24-RFC loads the 9-1-1 checkpoint clamp onto a recessed 5' end by binding the 5' DNA on Rad24 at an external surface site and threading the 3' ssDNA into the well-established internal chamber and into 9-1-1. We find here that Rad24-RFC loads 9-1-1 onto DNA gaps in preference to a recessed 5' DNA end, thus presumably leaving 9-1-1 on a 3' ss/ds DNA after Rad24-RFC ejects from the 5' gap end and may explain reports of 9-1-1 directly functioning in DNA repair with various TLS polymerases, in addition to signaling the ATR kinase. To gain a deeper understanding of 9-1-1 loading at gaps we report high-resolution structures of Rad24-RFC during loading of 9-1-1 onto 10-nt and 5-nt gapped DNAs. At a 10-nt gap we captured five Rad24-RFC-9-1-1 loading intermediates in which the 9-1-1 DNA entry gate varies from fully open to fully closed around DNA using ATPγS, supporting the emerging view that ATP hydrolysis is not needed for clamp opening/closing, but instead for dissociation of the loader from the clamp encircling DNA. The structure of Rad24-RFC-9-1-1 at a 5-nt gap shows a 180° axially rotated 3'-dsDNA which orients the template strand to bridge the 3'- and 5'- junctions with a minimum 5-nt ssDNA. The structures reveal a unique loop on Rad24 that limits the length of dsDNA in the inner chamber, and inability to melt DNA ends unlike RFC, thereby explaining Rad24-RFC's preference for a preexisting ssDNA gap and suggesting a direct role in gap repair in addition to its checkpoint role.
- Department of Structural Biology, Van Andel Institute, Grand Rapids, Michigan, USA.
Organizational Affiliation: