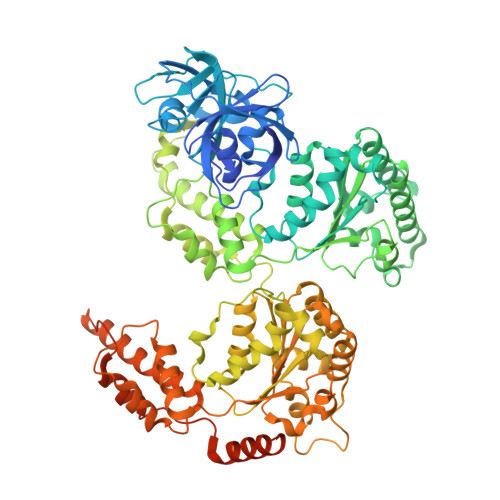
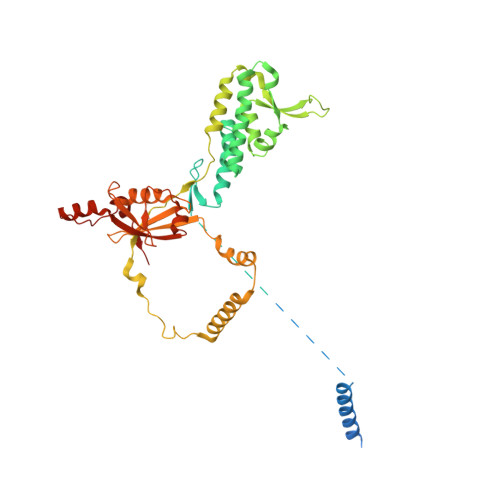
The p97/VCP adaptor UBXD1 drives AAA+ remodeling and ring opening through multi-domain tethered interactions.
Braxton, J.R., Altobelli, C.R., Tucker, M.R., Tse, E., Thwin, A.C., Arkin, M.R., Southworth, D.R.(2023) Nat Struct Mol Biol 30: 2009-2019
- PubMed: 37945741
- DOI: https://doi.org/10.1038/s41594-023-01126-0
- Primary Citation of Related Structures:
8FCL, 8FCM, 8FCN, 8FCO, 8FCP, 8FCQ, 8FCR, 8FCT - PubMed Abstract:
p97, also known as valosin-containing protein, is an essential cytosolic AAA+ (ATPases associated with diverse cellular activities) hexamer that unfolds substrate polypeptides to support protein homeostasis and macromolecular disassembly. Distinct sets of p97 adaptors guide cellular functions but their roles in direct control of the hexamer are unclear. The UBXD1 adaptor localizes with p97 in critical mitochondria and lysosome clearance pathways and contains multiple p97-interacting domains. Here we identify UBXD1 as a potent p97 ATPase inhibitor and report structures of intact human p97-UBXD1 complexes that reveal extensive UBXD1 contacts across p97 and an asymmetric remodeling of the hexamer. Conserved VIM, UBX and PUB domains tether adjacent protomers while a connecting strand forms an N-terminal domain lariat with a helix wedged at the interprotomer interface. An additional VIM-connecting helix binds along the second (D2) AAA+ domain. Together, these contacts split the hexamer into a ring-open conformation. Structures, mutagenesis and comparisons to other adaptors further reveal how adaptors containing conserved p97-remodeling motifs regulate p97 ATPase activity and structure.
- Graduate Program in Chemistry and Chemical Biology, University of California San Francisco, San Francisco, CA, USA.
Organizational Affiliation: