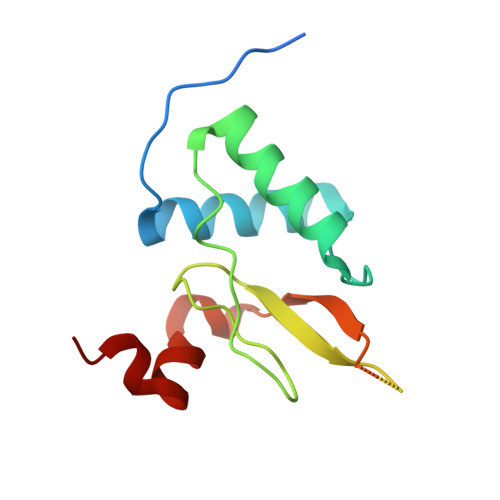
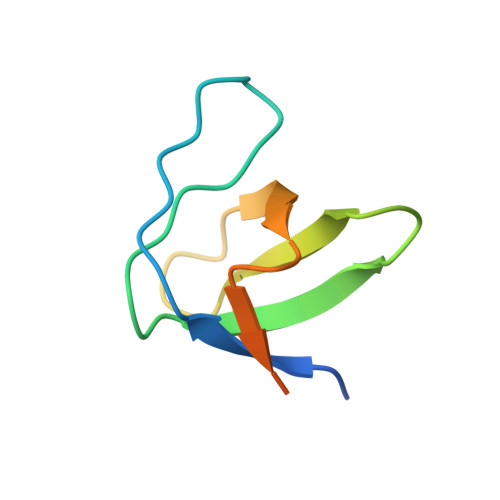
Neutron Reflectometry and Molecular Simulations Demonstrate HIV-1 Nef Homodimer Formation on Model Lipid Bilayers.
Heinrich, F., Thomas, C.E., Alvarado, J.J., Eells, R., Thomas, A., Doucet, M., Whitlatch, K.N., Aryal, M., Losche, M., Smithgall, T.E.(2023) J Mol Biology 435: 168009-168009
- PubMed: 36773691
- DOI: https://doi.org/10.1016/j.jmb.2023.168009
- Primary Citation of Related Structures:
8F2P - PubMed Abstract:
The HIV-1 Nef protein plays a critical role in viral infectivity, high-titer replication in vivo, and immune escape of HIV-infected cells. Nef lacks intrinsic biochemical activity, functioning instead through interactions with diverse host cell signaling proteins and intracellular trafficking pathways. Previous studies have established an essential role for Nef homodimer formation at the plasma membrane for most if not all its functions. Here we combined neutron reflectometry of full-length myristoylated Nef bound to model lipid bilayers with molecular simulations based on previous X-ray crystal structures of Nef homodimers. This integrated approach provides direct evidence that Nef associates with the membrane as a homodimer with its structured core region displaced from the membrane for partner protein engagement. Parallel studies of a dimerization-defective mutant, Nef-L112D, demonstrate that the helical dimerization interface present in previous crystal structures stabilizes the membrane-bound dimer. X-ray crystallography of the Nef-L112D mutant in complex with the SH3 domain of the Nef-associated host cell kinase Hck revealed a monomeric 1:1 complex instead of the 2:2 dimer complex formed with wild-type Nef. Importantly, the crystal structure of the Nef-L112D core and SH3 interface are virtually identical to the wild-type complex, indicating that this mutation does not affect the overall Nef fold. These findings support the intrinsic capacity of Nef to homodimerize at lipid bilayers using structural features present in X-ray crystal structures of dimeric complexes.
- Department of Physics, Carnegie Mellon University, Pittsburgh, PA 15213, USA; NIST Center for Neutron Research, Gaithersburg, MD 20899, USA.
Organizational Affiliation: