Structures of the R-type human Ca v 2.3 channel reveal conformational crosstalk of the intracellular segments.
Yao, X., Wang, Y., Wang, Z., Fan, X., Wu, D., Huang, J., Mueller, A., Gao, S., Hu, M., Robinson, C.V., Yu, Y., Gao, S., Yan, N.(2022) Nat Commun 13: 7358-7358
- PubMed: 36446785
- DOI: https://doi.org/10.1038/s41467-022-35026-6
- Primary Citation of Related Structures:
8EPL, 8EPM - PubMed Abstract:
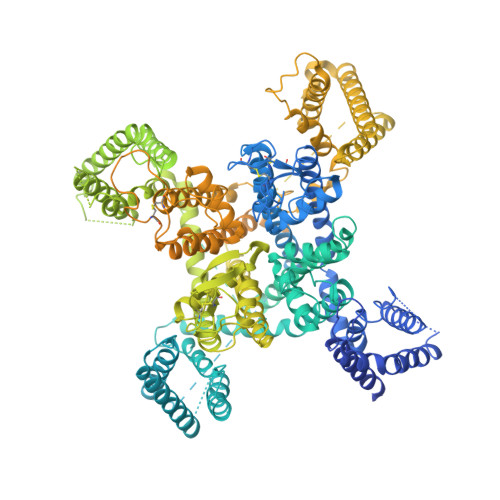
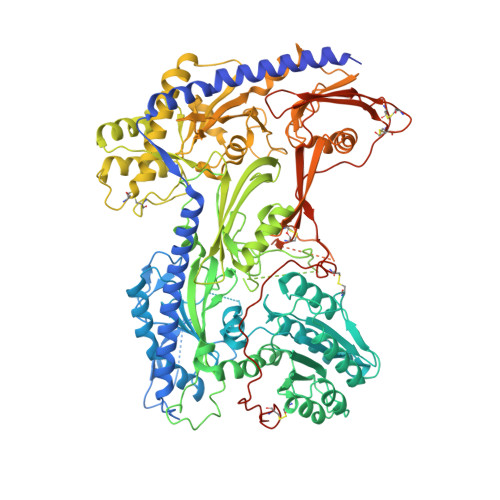
The R-type voltage-gated Ca 2+ (Ca v ) channels Ca v 2.3, widely expressed in neuronal and neuroendocrine cells, represent potential drug targets for pain, seizures, epilepsy, and Parkinson's disease. Despite their physiological importance, there have lacked selective small-molecule inhibitors targeting these channels. High-resolution structures may aid rational drug design. Here, we report the cryo-EM structure of human Ca v 2.3 in complex with α2δ-1 and β3 subunits at an overall resolution of 3.1 Å. The structure is nearly identical to that of Ca v 2.2, with VSD II in the down state and the other three VSDs up. A phosphatidylinositol 4,5-bisphosphate (PIP2) molecule binds to the interface of VSD II and the tightly closed pore domain. We also determined the cryo-EM structure of a Ca v 2.3 mutant in which a Ca v 2-unique cytosolic helix in repeat II (designated the CH2 II helix) is deleted. This mutant, named ΔCH2, still reserves a down VSD II , but PIP2 is invisible and the juxtamembrane region on the cytosolic side is barely discernible. Our structural and electrophysiological characterizations of the wild type and ΔCH2 Ca v 2.3 show that the CH2 II helix stabilizes the inactivated conformation of the channel by tightening the cytosolic juxtamembrane segments, while CH2 II helix is not necessary for locking the down state of VSD II .
- Department of Molecular Biology, Princeton University, Princeton, NJ, 08544, USA.
Organizational Affiliation: