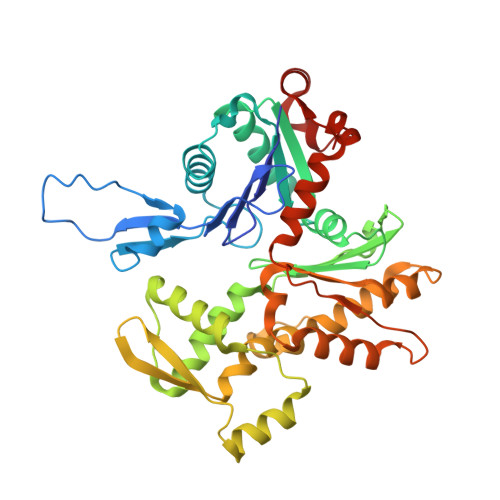
Myosin loop-4 is critical for optimal tropomyosin repositioning on actin during muscle activation and relaxation.
Doran, M.H., Rynkiewicz, M.J., Pavadai, E., Bodt, S.M.L., Rasicci, D., Moore, J.R., Yengo, C.M., Bullitt, E., Lehman, W.(2023) J Gen Physiol 155
- PubMed: 36459134
- DOI: https://doi.org/10.1085/jgp.202213274
- Primary Citation of Related Structures:
8EFI, 8ENC - PubMed Abstract:
During force-generating steps of the muscle crossbridge cycle, the tip of the myosin motor, specifically loop-4, contacts the tropomyosin cable of actin filaments. In the current study, we determined the corresponding effect of myosin loop-4 on the regulatory positioning of tropomyosin on actin. To accomplish this, we compared high-resolution cryo-EM structures of myosin S1-decorated thin filaments containing either wild-type or a loop-4 mutant construct, where the seven-residue portion of myosin loop-4 that contacts tropomyosin was replaced by glycine residues, thus removing polar side chains from residues 366-372. Cryo-EM analysis of fully decorated actin-tropomyosin filaments with wild-type and mutant S1, yielded 3.4-3.6 Å resolution reconstructions, with even higher definition at the actin-myosin interface. Loop-4 densities both in wild-type and mutant S1 were clearly identified, and side chains were resolved in the wild-type structure. Aside from loop-4, actin and myosin structural domains were indistinguishable from each other when filaments were decorated with either mutant or wild-type S1. In marked contrast, the position of tropomyosin on actin in the two reconstructions differed by 3 to 4 Å. In maps of filaments containing the mutant, tropomyosin was located closer to the myosin-head and thus moved in the direction of the C-state conformation adopted by myosin-free thin filaments. Complementary interaction energy measurements showed that tropomyosin in the mutant thin filaments sits on actin in a local energy minimum, whereas tropomyosin is positioned by wild-type S1 in an energetically unfavorable location. We propose that the high potential energy associated with tropomyosin positioning in wild-type filaments favors an effective transition to B- and C-states following release of myosin from the thin filaments during relaxation.
- Department of Physiology & Biophysics, Boston University Chobanian & Avedisian School of Medicine, Boston, MA.
Organizational Affiliation: