Cryo-EM structure of the HOPS core complex and its implication for SNARE assembly
Port, S.A., Farrell, P.D., Jeffrey, P.D., DiMaio, F., Hughson, F.M.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Vacuolar protein sorting-associated protein 33 | 696 | Thermochaetoides thermophila | Mutation(s): 0 | 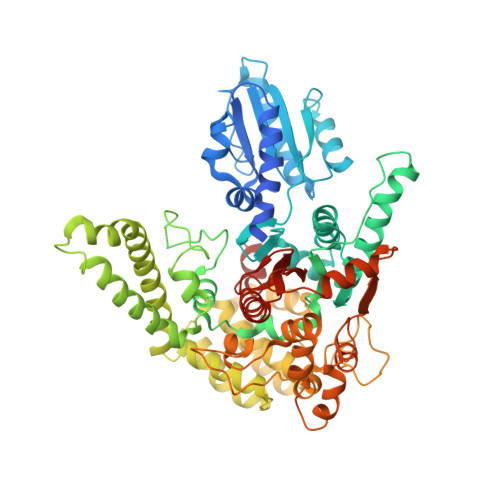 | |
UniProt | |||||
Find proteins for G0SCM5 (Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719)) Explore G0SCM5 Go to UniProtKB: G0SCM5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | G0SCM5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Vacuolar protein sorting-associated protein 16 | 834 | Thermochaetoides thermophila | Mutation(s): 0 | 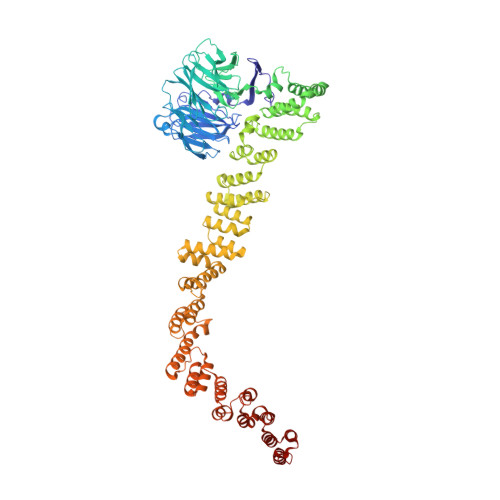 | |
UniProt | |||||
Find proteins for G0S6M7 (Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719)) Explore G0S6M7 Go to UniProtKB: G0S6M7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | G0S6M7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Vacuolar protein sorting-associated protein 18 | 968 | Thermochaetoides thermophila | Mutation(s): 0 | 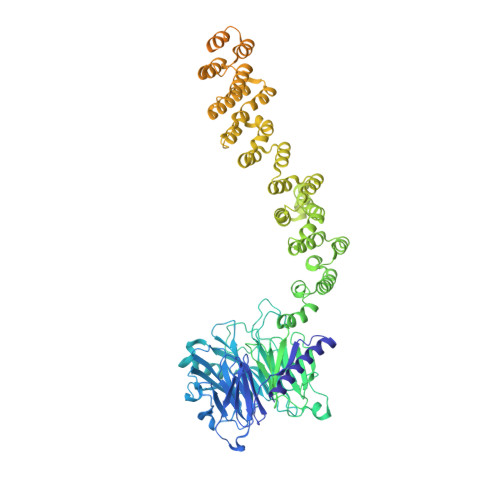 | |
UniProt | |||||
Find proteins for G0S4R9 (Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719)) Explore G0S4R9 Go to UniProtKB: G0S4R9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | G0S4R9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | cryoSPARC | 2.15.0 |
| RECONSTRUCTION | RELION | 3.1.0 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| German Research Foundation (DFG) | Germany | PO2195/1-1 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | R01GM071574 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | R01GM123089 |